Emerging treatments for advanced dry AMD
Investigational pipeline provides promise for an unmet need.

After 10 to 15 years of great advances in treatment for patients with exudative age-related macular degeneration (eAMD), many companies are moving to the forefront with research targeting the development of treatment for dry AMD.
The lack of therapy to halt the progression of dry AMD, including geographic atrophy (GA), represents a significant unmet need considering its effect on vision and the number of people affected. The Beaver Dam Eye Study found that 42% of eyes with GA were legally blind, and as the prevalence of GA increases dramatically from 4.4% among persons 85 years and older to 22% after age 90 years, the burden of GA can be expected to grow with our aging population.1,2
Fortunately, the hunt for a treatment for dry AMD is beginning to turn up positive strategies: neuroprotection, reducing oxidative stress and accumulation of toxic byproducts, visual cycle modulation, and use of stem cell therapy. Suppressing inflammation, however, has garnered the greatest interest.
Multiple compounds that suppress inflammation by inhibiting the complement pathway are under investigation in clinical trials. The rationale for this approach comes from the finding of activated complement components and complement regulators in histopathological drusen specimens and research documenting a genetic link between the complement system and AMD.
Of the complement inhibitors being studied, two have completed phase 3 clinical trials with positive results: pegcetacoplan (formerly APL2-103; Apellis Pharmaceuticals) and avacincaptad pegol (Zimura; Iveric Bio).
Pegcetacoplan is a pegylated, highly selective peptide that binds C3, preventing its cleavage. Inhibition of C3 prevents events in the complement cascade needed for opsonization, inflammation, and formation of the membrane attack complex (MAC) that leads to cell death.
Pegcetacoplan was investigated as a treatment for GA secondary to AMD in 2 global phase 3 studies, OAKS (NCT03525613) and DERBY (NCT03525600). These identically designed, double-masked studies have a planned 24-month duration and randomized approximately 1250 patients 2:2:1:1 to intravitreal injections of pegcetacoplan monthly, pegcetacoplan every other month (EOM), sham monthly, or sham EOM.
The primary end point assessing change in total area of GA lesions was assessed at month 12 and was met by both regimens of pegcetacoplan in OAKS (monthly: 22% reduction vs sham, P = .0003; EOM: 16% reduction, P = .0052) and by neither regimen in DERBY (monthly: 12% reduction, P = .0528; EOM: 11%; P = .0750). A prespecified pooled data analysis showed statistical superiority of pegcetacoplan versus sham for both regimens (monthly: 17%, P = .0012; EOM: 14% reduction, P < .0001) (Figure 1). In addition, whereas OAKS and DERBY entered patients with extrafoveal or subfoveal lesions, findings from prespecified subgroup analyses showed greater efficacy of pegcetacoplan among patients with extrafoveal lesions, which tend to grow faster than the subfoveal lesions. In a pooled analysis, extrafoveal GA growth was reduced 26% by pegcetacoplan monthly versus sham (P < .0001) and 23% for the EOM regimen (P = .0002).
Figure 1. Pegcetocoplan effect on lesion growth in prespecified combined analyses (OAKS and DERBY)
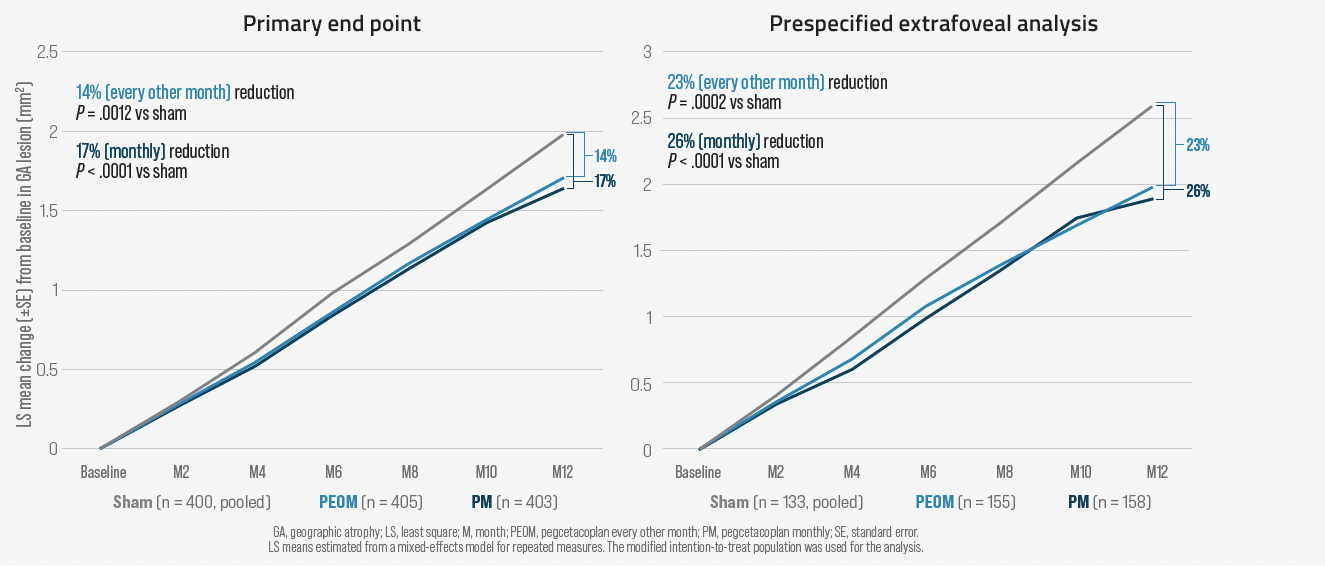
Safety data for pegcetacoplan were encouraging. Adverse events were less frequent overall than in the phase 2 FILLY study (NCT02503332), and only 8 serious ocular treatment-emergent adverse events (< 1%) occurred among eyes treated with pegcetacoplan.
Early cases of intraocular inflammation (IOI; n = 4), including a case of noninfectious endophthalmitis, were linked to drug impurity that was addressed through a change in manufacturing. In total, investigators observed only 14 cases of IOI; none of those involved vasculitis or occlusive vasculitis, and pegcetacoplan treatment was successfully resumed without IOI recurrence in 10 of the 13 patients (1 patient experienced 2 cases of IOI).
Exudative AMD (eAMD) was identified in 6.4% of eyes in the monthly pegcetacoplan group, 5% of eyes dosed EOM, and 3.8% of sham eyes. This rate includes investigator-determined eAMD and reading centerdetermined eAMD based on fluorescein angiography taken at month 12. This rate is much lower than that of the phase 2 FILLY trial, although the latter study included follow-up to 18 months. The experience during the OAKS and DERBY studies remains to be seen.
Avacincaptad pegol is a pegylated RNA aptamer that is a potent and specific inhibitor of C5, acting to block cleavage of C5 and thereby MAC formation. Investigators evaluated the agent in a completed phase 2b/3 study, GATHER 1 (NCT02686658) and a second pivotal trial (GATHER 2, NCT04435366) is ongoing with results expected in November 2022.
Figure 2. Avacincaptad pegol 2 mg vs sham treatment
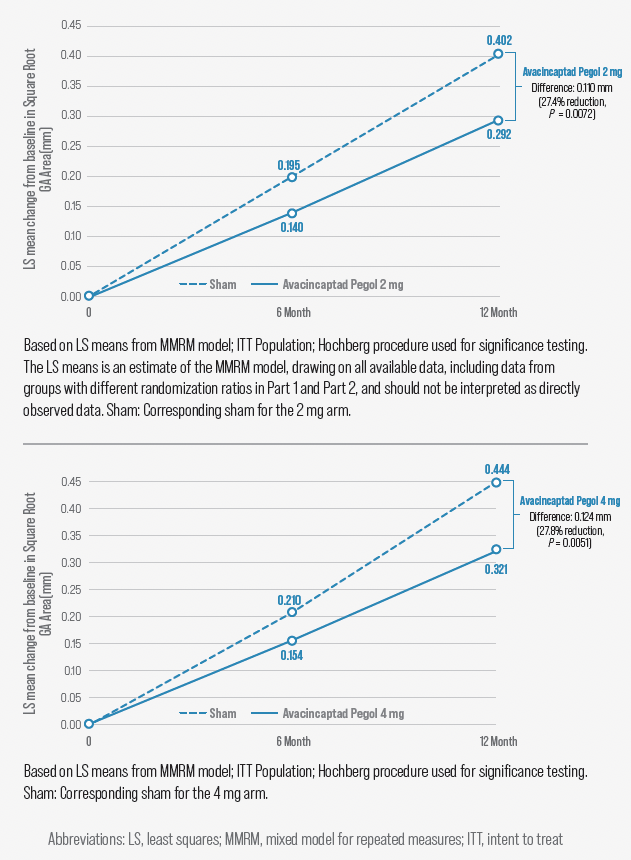
The completed study enrolled only patients with extrafoveal lesions and randomized them to monthly avacincaptad pegol 2 mg, avacincaptad pegol 4 mg, or sham. Both doses of avacincaptad pegol met the primary end point, showing statistically significant reduction in mean rate of GA growth (square root transformation) compared with sham (2 mg: 27.4% reduction, P = .0072; 4 mg: 27.8% reduction, P = .0051) (Figure 2). The benefit of avacincaptad pegol in treating extrafoveal GA is like that observed for pegcetacoplan in DERBY and OAKS.
Avacincaptad pegol was well tolerated. New onset of exudation occurred in approximately 9.3% of eyes treated with avacincaptad pegol versus 2.7% of control eyes. There were no ocular serious adverse events, cases of endophthalmitis, or inflammation in the avacincaptad groups.
The only other treatment for dry AMD that has advanced into a phase 3 trial is a commercially available oral formulation of low-dose doxycycline (Oracea; Galderma). Results are awaited from the TOGA trial (NCT01782989) investigating the rate of change in GA size in 286 patients randomized 1:1 after a 6-month observation phase to doxycycline 40 mg or placebo once daily.
It is well known that tetracyclines at low doses have anti-inflammatory and antioxidative properties that could be relevant for treating dry AMD. Furthermore, minocycline has been shown to protect human retinal pigment epithelial cells against oxidative damage and attenuate photoreceptor degeneration in a bright light model in rats.3,4
In addition, doxycycline was shown at low levels to reduce neovascularization and lesion volume in a rat model of laser-induced choroidal neovascularization.5
Future directions
Although complement inhibition appears to be a promising strategy for preventing progression of dry AMD, many unanswered questions exist regarding the efficacy and safety of this approach. For example, investigators do not know the source of complement activation, the involvement of the 3 complement pathways in AMD pathogenesis, whether an optimal site to block the complement cascade exists, and whether local treatment is an option. In addition, will complement inhibition require lifelong therapy? Are there associated long-term risks? Is there a role for combining treatments with different mechanisms of action?
Even though treatment that effectively reduces progression of GA can prevent visual loss in the long term, it remains to be seen how well intervention involving monthly or bimonthly injections will be accepted by patients who likely still have excellent vision despite their pathology.
Nevertheless, this is an exciting time for retinal specialists, as it appears practitioners are on the brink of a new era in which they will be able to offer treatment that will reduce the risk of visual loss for an important subset of patients with AMD.
David S. Boyer, MD
E: vitdoc@aol.com
David S. Boyer, MD, is a senior partner at Retina-Vitreous Associates Medical Group, California. Boyer is a consultant and/or investigator for Apellis Pharmaceuticals, Iveric Bio, and other companies developing treatments for dry age-related macular degeneration.
References
1. Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182-191.
2. Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112:787-798. doi:10.1016/j. ophtha.2004.11.040
3. Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MOM. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45(8):2753-2759. doi:10.1167/iovs.03-1344
4. Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C, Langmann T. Minocycline counterregulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. 2015;12:209. doi:10.1186/s12974-015-0431-4
5. Samtani S, Amaral J, Campos MM, Fariss RN, Becerra SP. Doxycycline-mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50(11):5098-5106. doi:10.1167/ iovs.08-3174

