The country has a goal of ensuring that more than 95% of patients with diabetes receive timely retinal examinations.

The country has a goal of ensuring that more than 95% of patients with diabetes receive timely retinal examinations.
The lesion area growth was reduced by more than 50% in the first human clinical trial of K8.

Researchers will investigate a gene in the eye that is crucial for normal vision, but can cause retinal diseases when mutated that often lead to blindness.
Findings from the retrospective, observational study support the potential anti-inflammatory benefits of the drug class.

The committee will work to help the organization increase awareness and early identification of diabetes-related eye diseases and improve access to eye care treatment, policy development, research, and surveillance.
Researchers used adaptive optics scanning light ophthalmoscopy (AOSLO) to study retinas in mice with photoreceptor damage, specifically photoreceptor laser injury.

A new study highlights the effectiveness of ERG/pupillometry in predicting diabetic retinopathy progression, surpassing traditional imaging methods.
New research identifies key factors influencing visual recovery in Leber hereditary optic neuropathy patients treated with gene therapy, enhancing treatment strategies.
Clearside Biomedical secures Health Canada approval for Xipere, while exploring strategic options to enhance its suprachoroidal delivery platform and pipeline.

New tariffs threaten the optical industry, prompting urgent reevaluation of sourcing strategies and pricing as deadlines approach for affected countries.

The report highlights clinical successes, innovation, research, and patient outcomes.

Researchers hypothesize that lamivudine was effective in the clinical trial against DME because the drug blocks the activity of inflammasomes.
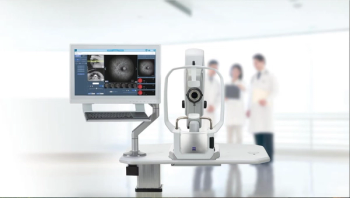
CLARUS 700, the advanced retinal imaging system, captures 133 degrees in a single image and up to 267 degrees with multiple images.

In a recent study, the researchers suggested that targeting the circuits responsible for visual acuity may be necessary in achieving optimal recovery of visual function and could aid in the development of vision restoration therapies.

The cross sector initiative that intends to drive a shift from reactive to proactive health care by leveraging ocular data through the means of oculomics.

The system was taught quality standards by being fed more than 1400 images from different parts of the retina taken by adaptive-optics ophthalmoscopy.

The recent study details the diagnostic value of a multimodal model utilizing both types of biomarkers when compared to unimodal models.
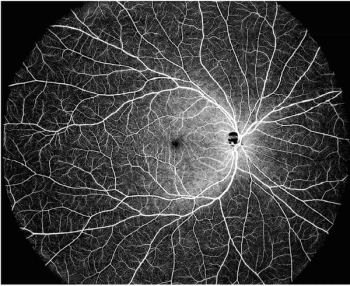
DREAM is an acronym for deep imaging depth, rapid sweeping speed, extensive scan range, accurate results, and multimodal imaging capabilities.

In total, 613,690 images used for screening were included in the study.

Valeda met the primary end point in the US LIGHTSIDE III trial, improving the best corrected visual acuity in patients for 24 months of >5 letters or equivalent to 1 line improvement on the eye chart, according to the company.
The recombinant human vascular endothelial growth factor receptor (VEGFR)-antibody human complement receptor 1 (CR1) fusion protein will be intravitreally administered in up to 150 patients.

The trial assesses GAL-101 as an oral therapy designed to treat Alzheimer disease, dry age-related macular degeneration, and glaucoma.

Industry leaders discuss the impact of rising tariffs on vision care costs, advocating for policy changes to ensure affordable access to essential eyewear.


The company also reported a positive outcome of an analysis of masked data from its ongoing MAGNIFY Phase 2 trial for zervimesine in adults with GA.

The announcement comes after a request was made by the Canadian Investment Regulatory Organization (CIRO) to explore the possibility of a sale.

The NPI-001 (N-acetylcysteine amide) tablets are a proprietary investigational therapy for the treatment of patients with retinitis pigmentosa.

The company's lead product candidate Duravyu (vorolanib intravitreal insert), f/k/a EYP-1901, is anticipating topline data from several clinical trials in 2026.

Published: August 7th 2025 | Updated:

Published: September 13th 2025 | Updated:

Published: January 26th 2024 | Updated:
Published: September 21st 2025 | Updated:

Published: August 15th 2025 | Updated: