
Optoretinography is an emerging technology used to test light-evoked photoreceptor activity.

Optoretinography is an emerging technology used to test light-evoked photoreceptor activity.

Imaging and biomarkers are driving earlier, more individualized GA treatment

The JADE clinical study enrolled more than 160 patients with diabetic macular edema (DME) or wet (neovascular) age-related macular degeneration (wAMD).

When no retina podcast was available, Jayanth Sridhar, MD, created one—and learned lessons about branding, bandwidth, and mentorship along the way.

Researchers uncover a DNA repair mechanism in Greenland sharks that preserves their vision for centuries, offering insights into longevity and eye health.

Strategies for treatment intervals, drug selection, and patient expectations.

David S. Boyer, MD, outlines changes in GA evaluation, the role of complement inhibition, monitoring for neovascular conversion, and emerging therapies.

Nanoscope's new patent enhances its innovative MCO technology, promising vision restoration for retinal degeneration with strong safety results and market potential.

A recent study represents a step forward in investigations of the retinal layers as they are affected by glaucoma.
The Part B dose-expansion portion is evaluating SB-007 for the treatment of Stargardt disease.

The Investigational New Drug (IND) application from Complement Therapeutics for CTx001 was previously approved by the FDA in October 2025.

A trio of retina specialists recently reviewed the clinical benefits of aflibercept 8 mg, including its extended dosing intervals, improved patient satisfaction, and enhanced treatment outcomes for various conditions.

Knowing what’s on the market for AMD and GA aids in preserving vision.

The pandemic reshapes treatment intervals for neovascular AMD, revealing flexible strategies that maintain visual outcomes and enhance patient care.
The novel system converts light into electrical signals to stimulate retinal cells.

The company did not receive the necessary stockholder votes to approve the merger agreement with Alcon at the Special Meeting of Stockholders held on January 6, 2026.

Advancements in macular laser therapy, emphasizing standardization, precision delivery, and innovative treatment strategies for retinal conditions.


For Jordan M. Graff, MD, FACS, early adoption is about reigniting the thrill of discovery and navigating the rewards, risks, and realities of translating innovation into practice.

The FDA noted in the letter that it is unable to approve the application for ONS-5010/LYTENAVA (bevacizumab-vikg) in its current form for the treatment of wet AMD.


The value of attending industry meetings at the beginning of a career in ophthalmology.

Discover key insights from the AAO 2025 meeting, highlighting advancements in real-world data, gene therapies, multitargeting treatments, and AI in retina care.

AI revolutionizes ophthalmology with enhanced diagnostics, personalized treatments, and improved surgical precision, offering better patient outcomes and efficient clinical trials.

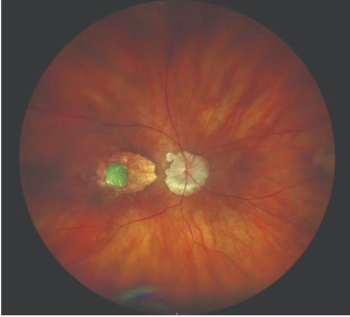
This approach helps distinguish diseases associated with macular atrophy.

The EU-ROP registry revolutionizes retinopathy of prematurity management, offering insights into treatment variations and outcomes across Europe.

Atrophy Advisor aids physicians in personalizing treatment for geographic atrophy in age-related macular degeneration, enhancing patient care and outcomes.

Dr. Quan Dong Nguyen presents interim data from the phase 3 DRAGON study on a potential first therapy for adolescent Stargardt disease.

Explore the evolving challenges and insights in long-term follow-up for pediatric gene therapy, focusing on data integrity, ethical considerations, and patient retention.

Lock in patient adherence with discussion of study results.