
Arctic Vision has a commercial collaboration agreement with Santen Pharmaceutical Co., Ltd. to commercialize ARVN001 for the treatment of UME.

Arctic Vision has a commercial collaboration agreement with Santen Pharmaceutical Co., Ltd. to commercialize ARVN001 for the treatment of UME.

The researchers successfully tested the robot using enucleated pig eyes.
The imaging system is designed to enable secure, real-time remote monitoring around the clock, from virtually any location.

This research project, titled, “A Generalizable Deep Learning Platform for Unifying Quantification of Experimental Autoimmune Uveitis.”

Prevent Blindness will be sharing a variety of free educational resources to the public and professionals during the awareness week, which will be held February 24, 2025, through March 2, 2025.
The topline results from the COAST trial is anticipated in early Q2 of 2025.

PST-611, a first in class non-viral vectorized therapy expressing human transferrin, a highly potent iron regulator.

OCU410 is a novel multifunctional modifier gene therapy candidate that targets multiple pathways associated with GA.

The submission deadline for both the 2025 Jenny Pomeroy Award for Excellence in Vision and Public Health and the sixth annual Rising Visionary Award is Monday, March 10, 2025, at noon ET.

A recent study of AEYE-DS AI technology used in conjunction with the Topcon NW500 resulted in exceptional clinical diagnostic efficacy.

Donald Munoz has over 30 years of health care experience as a chief financial officer and an investment banker.

David S. Boyer, MD will present data from the sozinibercept Phase 2b wet AMD trial.
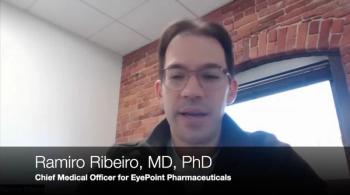
Ramiro Ribeiro, MD, PhD, shares insights from the ongoing research tyrosine kinase inhibitors for AMD and DME.
In the 5 patients with bilateral geographic atrophy (GA) who received a K8 implant in 1 eye, there was a mean reduction in GA lesion growth of 66% at 3 months.

InflammX’s pipeline includes an orally dosed therapeutic candidate targeting intermediate age-related macular degeneration.

The objective of this trial was to evaluate safety and tolerability and identify dose level for further evaluation.
OCU400 demonstrated meaningful improvement of 2-line gain (10 letters on ETDRS chart) in low-luminance visual acuity (LLVA) in treated eyes when compared to untreated fellow eyes.

City Therapeutics will develop a novel RNAi clinical candidate toward a specific disease target for intravitreal administration.

This phase 2b study (ASPIRE) is currently underway.

The application was refiled following a December 20, 2024, meeting between the US Food and Drug Administration (FDA) and Astellas.

Several pharmaceutical companies have announced new appointments of executives and board members as they prepare for growth in 2025.

Catch up on the clinical trial news, updates from the United States Food and Drug Administration (FDA), and company funding that you may have missed.

The collaboration between KIST and Huons BioPharma will span 14 months.

Most recently, Jacques served as Vice President of Healthcare Strategy, Partnerships & M&A at Microsoft.

The potential gene therapy candidate is being evaluated for geographic atrophy.

The conference will be held January 13-16, 2025 in San Francisco, CA.

Let’s look back on some of the top stories on Modern Retina from 2024 as we gear up for an incredible 2025.

Did you miss any of our print issues this year? Don’t worry, they are still available online.

Curacle plans to finalize its development strategy, including phase 2b and 3 trials, through a Type C meeting with the US FDA in February of 2025.

The publication is entitled, “Design and Characterization of a Novel Intravitreal Dual-Transgene Genetic Medicine for Neovascular Retinopathies.”