
Two FDA-approved geographic atrophy treatments show significant patient dropout, with 45% discontinuation by 18 months, driven by baseline vision, CNV, and lack of immediate visual improvement.

Two FDA-approved geographic atrophy treatments show significant patient dropout, with 45% discontinuation by 18 months, driven by baseline vision, CNV, and lack of immediate visual improvement.

Study reveals faricimab effectively treats diabetic macular edema in underrepresented minorities, showing significant visual acuity improvements and safety across diverse patient populations.

Ophthalmologist Dilraj Grewal explores cutting-edge clinical trials for uveitis treatment, highlighting new targeted therapies that aim to control inflammation and macular edema with fewer steroid-related side effects.

Lexitas Pharma Services' CMO discusses groundbreaking ophthalmology conference, emphasizing innovative clinical trials, emerging treatments for eye diseases, and the potential for transformative patient care strategies.

Retina specialist Priya Vakharia, MD, reveals promising ANX007 trial results, showing potential to prevent vision loss in geographic atrophy.

Ophthalmologist Tarek Hassan introduces new surgical approach for proliferative vitreoretinopathy, using precise small retinotomies instead of extensive retinal removal to improve patient outcomes.

Four-year study shows faricimab in treat-and-extend regimen maintains vision for diabetic macular edema patients with significantly fewer injections over time.

Kapil Mishra, MD, discusses an overview of radiation retinopathy and its challenges in treating uveal melanoma from his presentation "Radiation Retinopathy: Future Strategies,"

Duke professor Sharon Fekrat, MD, discusses AI-driven ophthalmology with smartphone diagnostics and robotic procedures, emphasizing global collaboration and innovative medical research.





At Retina World Congress 2025, Dilsher S. Dhoot, MD, shares updates on the HELIOS trial and the potential future of tyrosine kinase inhibitors (TKIs).



Companies working in the retina space have announced that data from clinical trials will be shared at this year’s meeting in Fort Lauderdale, Florida.

The findings of this model support KIO-104 as a promising therapeutic candidate for both the prevention and treatment of PVR.

The closing of the merger is expected to occur as soon as practicable, subject to the satisfaction or waiver of the remaining customary closing conditions.

The ReNEW study for dry AMD is evaluating the efficacy, safety, and pharmacokinetics of daily subcutaneous injections of elamipretide, wa first-in-class mitochondria-targeted investigational therapeutic.

Hashad is a retinal specialist with over 25 years of leadership experience in global research and development.
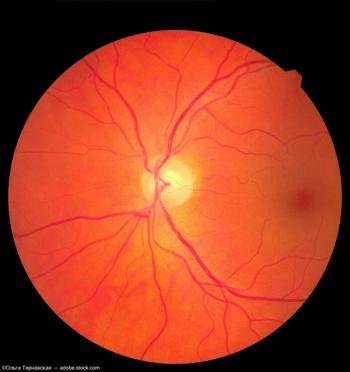
This database could enhance the workflow of eye care professionals with its in-depth reference data for retinal layer thickness and optic nerve head metrics.
The findings were from 6 patients in the first low-dose cohort of the ongoing first-in-human trial (NCT04627428).

Retina Resource was developed by retina specialists for retina specialists.

PYK-2101 is a first-in-class biodegradable retinal hydrogel sealant.

Apellis Pharmaceuticals and EyePoint Pharmaceuticals are both scheduled to present at the conference.

This follows successful Phase 1 results, which demonstrated a favorable safety profile for BI 771716 across both single and multiple intravitreal doses.

This device is designed to assist healthcare professionals in analyzing fundus images for the early detection of key retinal conditions.

The intent is that the board provide strategic guidance and expert insights to support the development of the company’s programs and science.
RPESC-RPE-4W is a cell product derived from adult retinal pigment epithelial stem cells.