
"What research at the 2023 ASRS meeting do you find exciting or interesting?" Here's what Aaron Lee, MD, Megan Baldwin PhD, and Carl Danzig, MD had to say!

"What research at the 2023 ASRS meeting do you find exciting or interesting?" Here's what Aaron Lee, MD, Megan Baldwin PhD, and Carl Danzig, MD had to say!
Priya Vakharia, MD, spoke with our team about her presentation at the Women in Ophthalmology Summer Symposium being held in Marco Island, Florida.

Sruthi Arepalli, MD, spoke with our team about the current landscape of geographic atrophy (GA) and her presentation at the Women in Ophthalmology Summer Symposium being held in Marco Island, Florida.

The 3D viewing systems and digital microscopes offer numerous advantages for performing the procedures.

Maria H. Berrocal spoke with our team about her presentation at the Women in Ophthalmology Summer Symposium being held in Marco Island, Florida.
Carl Regillo, MD, FACS, FASRS, spoke with our team following the annual ASRS meeting in Seattle, Washington to share insights from his presentation titled, "Modulation of Macrophages and Complement Dysfunction in Non-exudative AMD utilizing novel, sialic-acid coated nanoparticles." Aviceda Therapeutics is unlocking the proteogenomic code of AMD to target proteins and pathways linked to macular degeneration.

Kerrie Brady, Chief Executive Officer and President of OcuTerra Therapeutics shared updates from the company including the development of OTT166 at the 2023 ASRS annual meeting.

David Lally, MD, spoke with our team about the ZETA-1 phase 2 trial efficacy results for APX3330, a novel, oral ref-1 inhibitor for the treatment of diabetic retinopathy at the 2023 ASRS annual meeting.

Veena Raiji, MD, MPH, spoke with our team about long-term outcomes of eyes requiring IOP-lowering medication in the PALADIN trial at the 2023 ASRS annual meeting.

J. Fernando Arevalo, MD, PhD, FACS, FASRS, a member of Modern Retina's editorial advisory board, spoke with us about clinical characteristics of macular holes that close without surgery.

In addition to the educational offerings, the program offers opportunities for relaxation and spiritual and family-building events.

At the 2023 ASRS annual meeting in Seattle, Washington, Michael Singer, MD, spoke with our team about the Starlight Study, which is an optogenetic study looking at patients who had Stargardt disease.

Aleksandra Rachiskaya, MD, a member of the Modern Retina advisory board, spoke with our team about time to fluid control with faricimab arms complete compared to aflibercept in recent studies at the 2023 ASRS annual meeting.

Nancy Lurker, Executive Vice-Chair of the Board of EyePoint Pharmaceuticals, discussed the subgroup analyses of the Phase 1 DAVIO Trial of EYP-1901 with our team at the 2023 ASRS annual meeting.

Paul Hahn, MD, PhD, shared insights on research comparing the relative efficacy of pegcetacoplan versus avacincaptad pegol in patients with geographic atrophy presented at the 2023 ASRS annual meeting.

President and CEO at Outlook Therapeutics, Russ Trenary, shared an update on the company's work toward FDA approval for Bevacizumab with our team at the 2023 ASRS annual meeting.

Megan Baldwin, PhD, CEO and Managing Director of Opthea Limited, spoke with our team about the company's clinical trial drug OPT-302 (sozinibercept) at the 2023 ASRS annual meeting.

Tarek Hassan, MD, spoke with our team about a novel glyco-mimetic nanoparticle, Aviceda AVD-104, being developed for the treatment of geographic atrophy.

At the 2023 ASRS annual meeting in Seattle, Washington, Ursula Schmidt-Erfurth, MD, spoke with our team to share information on the GALE extension study.

The Modern Retina team caught up with one of our board members, Fernando Arevalo, MD, PhD, FACS, FASRS, who shared information on his presentation on the treatment of progressively symptomatic retinal detachments associated with retinoschisis cases at the 2023 ASRS annual meeting.

In the study, researchers found that nearly one-quarter of anti-VEGF injections were received at least 1 week later than intended.

Shawn Kavoussi, MD, shared insights from his research on using infrared video for vitreous opacities and comparing postoperative reduction in vitreous opacity scores using infrared video with the Heidelberg spectralis unit.
Matthew R. Starr, MD, discusses how secondary lens techniques have changed over time.

Durga Borkar, MD, MMCi, joined our team to discuss the FARETINA-DME study At the 2023 ASRS meeting in Seattle, Washington.
Richard B. Rosen, MD, shared insights from his presentation from ASRS 2023 on "New 'MacGyver-inspired' endolaser option for chandelier-assisted scleral buckles" with Sheryl Stevenson, our Group Editorial Director.
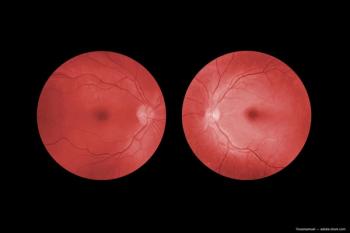
The research saw that new-onset full-thickness macular holes (FTMHs), especially those resulting from blunt ocular trauma, might close spontaneously without the need for a surgical intervention.

Cindy Cai, MD, shared insights from her presentation from ASRS 2023 on "Health disparities in lapses in diabetic retinopathy care" with Sheryl stevenson, our Group Editorial Director.

In presentations at the ASRS 41st Annual Meeting in Seattle, additional analyses support consistent protection from vision loss.

During presentations at the ASRS 41st Annual Meeting in Seattle, Apellis noted that there was no indication that drug product or manufacturing issues contributed to rare events of retinal vasculitis, and no events were reported in clinical trials.

Michael Ip, MD, shared his perspective on the 2023 ASRS meeting in Seattle, Washington. He noted presentations and trends that are key at this event and why he thinks they are valuable topics to understand and review for retina specialists.