
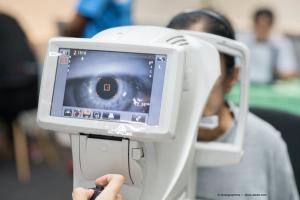
Optoretinography is an emerging technology used to test light-evoked photoreceptor activity.

Lynda Charters Enoch started her early “eye life” at the Schepens Eye Research Institute, Boston, which ultimately culminated in her current position as an Editor of Ophthalmology Times.

Optoretinography is an emerging technology used to test light-evoked photoreceptor activity.

Imaging and biomarkers are driving earlier, more individualized GA treatment

Researchers uncover a DNA repair mechanism in Greenland sharks that preserves their vision for centuries, offering insights into longevity and eye health.

Strategies for treatment intervals, drug selection, and patient expectations.

A recent study represents a step forward in investigations of the retinal layers as they are affected by glaucoma.
The novel system converts light into electrical signals to stimulate retinal cells.


The FDA noted in the letter that it is unable to approve the application for ONS-5010/LYTENAVA (bevacizumab-vikg) in its current form for the treatment of wet AMD.


AI revolutionizes ophthalmology with enhanced diagnostics, personalized treatments, and improved surgical precision, offering better patient outcomes and efficient clinical trials.

Atrophy Advisor aids physicians in personalizing treatment for geographic atrophy in age-related macular degeneration, enhancing patient care and outcomes.

Lock in patient adherence with discussion of study results.

Remote monitoring of age-related macular degeneration enhances patient care, reduces treatment burden, and leverages AI for personalized management.

Innovative multimodal deep learning models enhance non-invasive chronic kidney disease screening by integrating retinal images and urine dipstick data for improved accuracy.

The Portal extension trial reveals that the Port Delivery System significantly improves vision in AMD patients, showcasing long-term efficacy and durability.

New treatments show promise in preventing fibrosis in neovascular AMD, addressing a critical need for improved visual outcomes in patients.


In the study, authors investigated the natural history of GA lesion incidence rates and analyzed potential risk factors for faster incidence of GA lesions.

Emerging anti-VEGF agents offer enhanced durability and anatomic outcomes in retinal disease.

A study reveals that lesion characteristics in geographic atrophy significantly influence growth rates, highlighting the need for targeted treatment strategies.

Fovea-sparing, multifocal, and bilateral lesions exhibited the fastest growth rates.

A recent study reveals that systemic chemotherapy significantly reduces mortality and enucleation rates in retinoblastoma, enhancing patient outcomes.

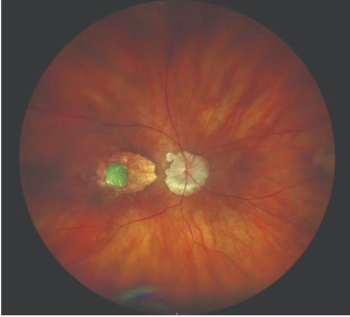
The investigators noted that this report is the first about subretinal drusenoid deposits in Black and Hispanic patients with age-related macular degeneration.

A recent study uncovers a novel biomarker for retinal vascular diseases, highlighting the significance of intermittent capillary perfusion in monitoring treatment efficacy.

Maximizing treatment based on GA location, lesion progression, and morphologic retinal changes.
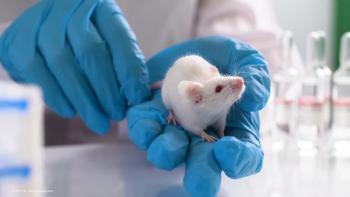
The procedure showed positive effects on the electroretinography (ERG) (b-wave, oscillatory potentials), optomotor response, and contrast sensitivity in actively treated eyes compared with controls.


Patient response focused on perceived vision-related quality of life outcomes, investigators said.

The trial results show promising for the evaluated gene therapy for the treatments of LCA, showing significant vision improvements in pediatric patients.
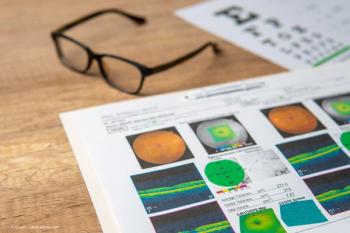
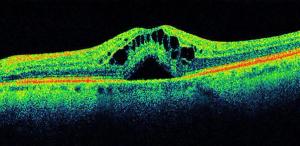
Published: July 26th 2020 | Updated:

Published: September 17th 2020 | Updated:

Published: June 28th 2021 | Updated:

Published: November 12th 2021 | Updated:

Published: September 9th 2021 | Updated:
Published: November 15th 2020 | Updated: