The platform to streamline clinical decision workflows, management of teleophthalmology, referral decisions and patient management while improving clinic efficiencies.

The platform to streamline clinical decision workflows, management of teleophthalmology, referral decisions and patient management while improving clinic efficiencies.

Rajendra Apte, MD, PhD, has received the Research to Prevent Blindness /American Macular Degeneration Foundation Catalyst Award for innovative research approaches in studying age-related macular degeneration.

iCRx is set to release the One Minute Eye Exam in April, potentially changing the way ophthalmologists and optometrists conduct eye examinations for corrective lenses.

The free, online event will feature presentations from Rachel Huckfeldt, MD, PhD, and Rachelle Lin, OD.
Twelve-week data from the Ph1b/2a AMARONE trial reveals Restoret to be well-tolerated in patients with diabetic macular edema and neovascular age-related macular degeneration.

According to a news release, the Redwood City, California, site will complement Eyenovia’s facility in Reno, Nevada as well as its contract manufacturer, Coastline International, to produce commercial supply of Mydcombi.

According to the organization, GYROS results will help researchers design clinical trials for an emerging gyrate atrophy gene therapy.
Researchers at the Eye Clinic of the University Hospital Bonn (UKB) and the University of Bonn have tested a new imaging method for monitoring intermediate uveitis.
Preliminary safety data support a favorable benefit-risk profile at both dose levels. The preliminary efficacy and safety data trending similar to or better than the OPTIC study, in which patients continue to see ongoing clinical benefit through at least 3 years.

OK-101 is the first IND clearance granted by the FDA for a drug to begin clinical studies specifically to treat patients suffering with neuropathic corneal pain.
Researchers noted a study by Ruhr-University Bochum researchers provides crucial evidence that the ECM influences visual sensory motor behavior already at the retina level, without intervention of disease and/or experimental manipulation.

Clearside noted in a news release it intends to use the net proceeds from this offering for working capital and general corporate purposes.

Toku’s AI technology, CLAiR, has obtained CE and UKCA Marks. CLAiR provides fast, accurate, non-invasive cardiovascular disease (CVD) risk assessments using standard retinal images.

According to the company, AGTC-501 was generally safe and well-tolerated and showed improvements in visual function at the 12-month analysis. The Phase 2/3 VISTA trial for AGTC-501 in XLRP expected to begin in in the first half of 2024.

According to the company, 4D-150 was well tolerated with a favorable safety profile when evaluated through up to 48 weeks of follow-up, with no significant inflammation observed.

A team of neuroscientists at Johns Hopkins show how specialized cells signal the presence of light simultaneously in two distinct ways.

The agreement settles Zeiss’s lawsuit against Topcon’s US-based subsidiaries that had been set to start this month in US federal court for the Northern District of California.
Trial will analyze efficacy, safety of AVD-104 versus avacincaptad pegol to treat GA

Under the terms of the agreement, Kiora Pharmaceuticals and Théa Open Innovation will develop and commercialize KIO-301 for the treatment of inherited retinal diseases in a deal valued at up to $301 million.

In a news release, the FDA noted these are copycat eye drop products that consumers can easily mistake for Bausch + Lomb’s Lumify brand eye drops, an over-the-counter product approved for redness relief.
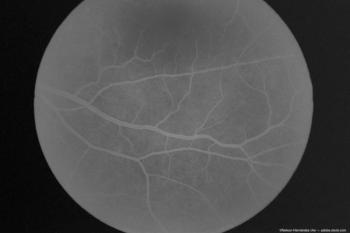
According to the company, the deals from two global Phase III RVO studies will be presented at Angiogenesis, Exudation, and Degeneration 2024.

Massachusetts Eye and Ear researchers came up with a mobile app called All_Aboard, designed to be used along with mainstream GPS systems and focuses on improving micro-navigation.

A University of Houston professor found that the excitement over the results of LLR as a treatment option for patients with myopia may have come too soon, before its efficacy was confirmed.
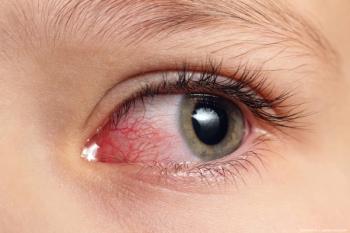
The licensing deal includes upfront, development milestones, and sales royalties, with additional considerations throughout the term of the agreement.

According to the company, Eyconis Inc. is developed with a $150 million commitment from external investors.

Sanders is affiliated with the Retina Group of Washington (RGW) and PRISM Vision Group. He is the first African American and the first PRISM-affiliated physician to serve as president of the ASRS.

LambdaVision is leveraging the microgravity environment of the space station to develop higher-quality implants than is currently possible on Earth.
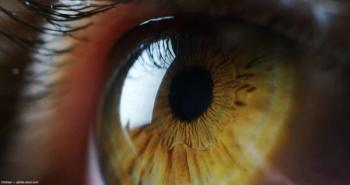
According to the company, ACDN-01 is the first-ever RNA exon editor to enter clinical development and the only clinical-stage therapeutic targeting the genetic cause of Stargardt disease. Ascidian expects to initiate enrollment in Phase 1/2 STELLAR study in the first half of 2024.

According to researchers at Johns Hopkins Children’s Center, AI-driven cameras take images of the back of the eye and require no eye drops can be used to close care gaps.