
Jennifer Lim, MD, FARVO, FASRS, discusses her ARVO presentation on sickle cell retinopathy, highlighting improvements in retinal surgery techniques and instrumentation.


Researchers develop AI system that clarifies images of RPE, making cells visible

Jennifer Lim, MD, FARVO, FASRS, discusses her ARVO presentation on sickle cell retinopathy, highlighting improvements in retinal surgery techniques and instrumentation.

Opus Genetics reveals promising 1-year results from OPGx-LCA5 gene therapy, showing sustained vision improvements in adults with LCA5 retinal degeneration.

The LUMEOS phase 3 clinical trial resulted in botaretigene sparoparvovec not meeting primary endpoints to improve visual navigation of XLRP.

Prevent Blindness has declared May the second annual inherited retinal disease and genetic testing month, offering free resources on education and other awareness efforts.


Administered via intravitreal injection, Synthopsin-MCO-010 possesses a promoter to specifically target ON-bipolar cells.

SpliceBio continues to actively enroll patients in the ASTRA study and accompanying POLARIS trial

ENCELTO is the first and only FDA-approved treatment for MacTel.

The modifier gene therapies from Ocugen target geographic atrophy and Stargardt disease.

Researchers indicated that pediatric patients with Leber congenital amaurosis experienced improvements in vision.

Researchers from the University of Bristol recorded increased instances of inflammation in particular demographic groups

The trial will feature the company’s product candidate, AXV101, focused on combating childhood blindness due to retinitis pigmentosa (RP) caused by Bardet-Biedl Syndrome 1 (BBS1).

Laru-zova is a gene therapy currently being investigated for the treatment of patients with X-linked retinitis pigmentosa.

Previous research has shown that voretigene neparvovec administered subretinally early in childhood for RPE65-mediated inherited retinal dystrophy achieved encouraging efficacious results.

The NPI-001 (N-acetylcysteine amide) tablets are a proprietary investigational therapy for the treatment of patients with retinitis pigmentosa.
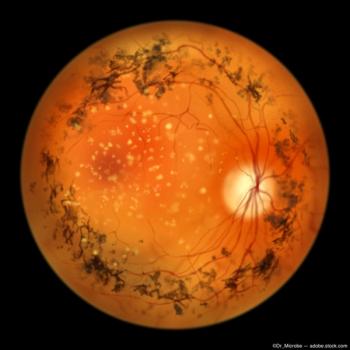
OCU400 demonstrated meaningful improvement of 2-line gain (10 letters on ETDRS chart) in low-luminance visual acuity (LLVA) in treated eyes when compared to untreated fellow eyes.

ViGeneron also received approval for dose escalation in the ongoing phase 1b clinical trial.

LHON, a genetic disease that affects the retinal ganglion cells, results in severe bilateral sequential vision loss.
The company is advancing its Phase I/II trial and exploring accelerated approval pathways in the US and Europe.

USC is leading a research team aiming to better understand retinitis pigmentosa and inform future treatments.

In the LIGHTHOUSE study, Atsena Therapeutics is evaluating ATSN-201 gene therapy for X-linked retinoschisis, leveraging AAV.SPR capsid for central retina transduction without foveal detachment risks.

Kaczmarek has a notable background of executive leadership and operational expertise in the gene therapy manufacturing industry.

This interview comes at the Children's Hospital Los Angeles team celebrates its 100th retinal gene therapy procedure.

The aim of this program is to help advance junior clinical research scientists in their professional endeavors to cure retinal degenerative disease.

Data show promising early improvements in low luminance visual acuity, a key measure of visual function.