
VISTA is a global randomly assigned, controlled, masked, multi-center pivotal study evaluating the efficacy, safety, and tolerability of 2 dose levels of AGTC-501 for the treatment of X-linked retinitis pigmentosa

VISTA is a global randomly assigned, controlled, masked, multi-center pivotal study evaluating the efficacy, safety, and tolerability of 2 dose levels of AGTC-501 for the treatment of X-linked retinitis pigmentosa

The trial evaluates OCU410ST, a modifier gene therapy candidate utilizing an AAV delivery platform for the retinal delivery of the RORA gene.

Researchers at Massachusetts Eye and Ear led a phase 1/2 trial, which included 14 participants, that found the experimental treatment was safe and efficacious.

Christine Kay, MD, spoke with Modern Retina to share insights from her presentation on longitudinal BCVA and safety analysis of mutation-agnostic MCO-010 optogenetic therapy For retinitis pigmentosa

The data will be presented in oral session at the 2024 American Society of Gene & Cell Therapy meeting May 7-11 in Baltimore, Maryland.

This initiative will provide a variety of tools to promote awareness and education, including some resources in both English and Spanish.

The Vision Simulator shows disease progression and vision loss simulations for diabetic macular edema, diabetic retinopathy, AMD, XLRP, and achromatopsia.

According to the company, SGT-1001 uses its novel gene coding technology for gene integration via transposition. It plans to advance SGT-1001 into the clinic in the first half of 2025. Preclinical data for SGT-1001 will be presented at the Association for Research in Vision and Ophthalmology and the American Society of Gene & Cell Therapy annual meetings.
According to the company, the grant funding from the Choroideremia Research Foundation is in support of validating functional vision assessments for patients with profound blindness.
According to the company, VG901 is designed to deliver a functional CNGA1 gene to retinal photoceptor target cells in patients diagnosed with retinitis pigmentosa. It has been granted Orphan Drug Designation by FDA.

According to the company, the Phase 3 study will have a sample size of 150 participants—one arm of 75 participants with the RHO gene mutation and the other arm with 75 participants that are gene agnostic.

Stargardt disease is the most common inherited retinal dystrophy (causing blurring or loss of central vision) in both adults and children.

The new cohort will add patients living with IRDs caused by mutations in multiple genes to the already existing cohort of patients with IRDs caused by single gene mutations.
In this cohort of 3 adult patients, there was positive safety and efficacy data.
MCO-010 demonstrated a statistically significant improvement of best-corrected visual acuity (BCVA) at week 52.

In a recent study led by Steven Pittler, PhD, and his team at the University of Alabama Birmingham (UAB), the role of modifier genes in retinitis pigmentosa type 59 (RP59) was meticulously examined.

Gildeuretinol halts Stargardt disease progression for up to 6 years.

In the study, published in Nature Biotechnology, researchers used their new system to correct disease-causing mutations in the eyes of two mouse models of genetic blindness, partially restoring their vision.

Lowe syndrome, a genetic disease, affects about 1 in 500,000 people in the general population.

According to the company, Stargardt disease is an orphan blindness disease that affects approximately 35,000 people in the United States.
The authors suggested genetic mutation may relax the body’s defences and allow harmful bacteria to reach the eye.

The treatment is a minimally invasive intravitreal injection that can be performed with a topical anesthetic.

A new initiative from Prevent Blindness will kick off to provide free educational resources on retinopathy of prematurity.
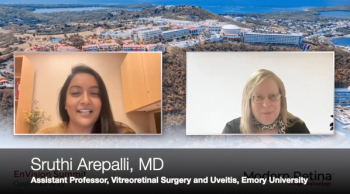
Sruthi Arepalli, MD, discusses the case of a patient with syphilis and sheds light on the complexities of diagnosing a masquerading syndrome.
The free, online event will feature presentations from Rachel Huckfeldt, MD, PhD, and Rachelle Lin, OD.