Retinal Surgery
Latest News
Latest Videos

CME Content
More News

Leanne Clevenger, MD, described a case that highlights varying approaches and protocols for addressing a complex case of retinal detachment repair in a patient with trauma to the globe.

David Hutton of Ophthalmology Times talks with Sunir Garg, MD about his surgical video panel at this year's Retina Fellows Forum.
According to the Advanced Research Projects Agency for Health, its program aims to transplant human eyes and reestablish visual connection to the brain.

Oliver Hvidt, CEO and co-founder of Norlase, spoke with Ophthalmology Times about the company's 4-year journey from AAO 2019 to present at this year's American Academy of Ophthalmology meeting.

NYU Langone Health performs landmark procedure called “major paradigm shift for potential vision therapies”

Launched in 2014, Intelligent Research in Sight is the nation's first and largest comprehensive eye disease registry.

According to the company, the software is designed to streamline the flow of information, surgical planning, and enable electronic medical record and diagnostic device integration.
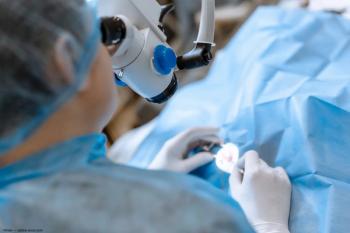
A modified endolaser setup was found to be safe and efficient for use during chandelier scleral buckling.

The new range of pure and premixed gases are intended for medium-term use in retinal surgery.

Our team spoke to Varun Chaudhary, MD, about his EURETINA presentation, titled "Systematic Review and Metanalysis in Macular Hole RCTs – Posturing after Macular Hole Surgery."
Despite its status as an essential postoperative safety eye product and its utilization by every ocular surgeon with every ocular surgery, eye shield innovation has been neglected to date.

According to the company, the deal expands its European footprint into Spain and Portugal.

EyeSouth Partners has partnered with Retina and Vitreous of Texas, a retina-only practice in Houston, Texas. The company’s current network encompasses 38 practices and over 300 doctors who deliver medical and surgical eye care services across more than 180 locations.
More than 135 patients have been recruited in 4 months for Phase 2 study of a single intravitreal (IVT) injection of ONL1204 ophthalmic solution as an adjunct to standard-of-care surgery.

WIO 2023: New developments in vitreoretinal surgery 3D viewing systems and robotics: The future Is now
The 3D viewing systems and digital microscopes offer numerous advantages for performing the procedures.

Maria H. Berrocal spoke with our team about her presentation at the Women in Ophthalmology Summer Symposium being held in Marco Island, Florida.

The FDA has assigned a Prescription Drug User Fee Act (PDUFA) action date for APP13007 of March 4, 2024.
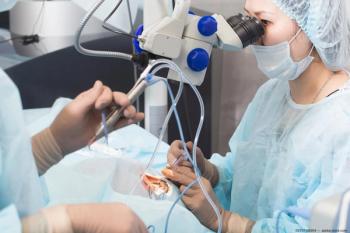
The plan is intended to upgrade Vitagrus’ manufacturing process so that it can ultimately handle the global market supply.
In this study, the investigators retrospectively reviewed all study patients who underwent pars plana vitrectomy with silicone oil tamponade for postsurgical endophthalmitis.

Oculis Holding AG announced, if approved, OCS-01 has the potential to become a new standard of care as the first once-daily, topical, preservative-free corticosteroid for treating inflammation and pain following ocular surgery.

J. Fernando Arevalo, MD, PhD, FACS, FASRS, a member of Modern Retina's editorial advisory board, spoke with us about clinical characteristics of macular holes that close without surgery.

ASRS 2023: Modern Retina board member, Fernando Arevalo, shares insights from his presentation on retinal detachments associated to retinoschisis
The Modern Retina team caught up with one of our board members, Fernando Arevalo, MD, PhD, FACS, FASRS, who shared information on his presentation on the treatment of progressively symptomatic retinal detachments associated with retinoschisis cases at the 2023 ASRS annual meeting.
Matthew R. Starr, MD, discusses how secondary lens techniques have changed over time.
Richard B. Rosen, MD, shared insights from his presentation from ASRS 2023 on "New 'MacGyver-inspired' endolaser option for chandelier-assisted scleral buckles" with Sheryl Stevenson, our Group Editorial Director.