
Boehringer Ingelheim announced that the phase 2 clinical studies will investigate a potential first-in-class oral compound and a highly specific antibody fragment for geographic atrophy.

Boehringer Ingelheim announced that the phase 2 clinical studies will investigate a potential first-in-class oral compound and a highly specific antibody fragment for geographic atrophy.
Topline results from the Magnify phase 2 clinical trial of oral zervimesine show 28.6% slower geographic atrophy lesion growth compared with placebo.
MonacoPro, the next evolution of Monaco from Optos, retains the powerful ultra-widefield SLO and spectral domain imaging while adding additional key product features.
The approval follows a refiling after the FDA issued a Complete Response Letter due to language on the amended label.

This year was brimming with advancements in optometry.

The agency approved Yesafili (Biocon Biologics) and Opuviz (Samsung Bioepis, Biogen) as biosimilars to Eylea (Regeneron Pharmaceuticals).

The approval marks the first new steroid on the ophthalmic market in more than 15 years.

The 2024 EnVision Summit will feature a brand-new Optometry Program, co-chaired by Danica Marrelli, OD, FAAO, AAO Dipl, and Cecelia Koetting, OD, FAAO, DipABO.

From FDA approvals to innovations in lens tech, 2023 was a big year in eye care.
Going forward, sozinibercept will be the official moniker of OPT-302, which holds fast track designation from the US Food and Drug Administration (FDA).
The submission of 24-month efficacy data from DERBY and OAKS is classified as a Major Amendment to the New Drug Application, which delays the PDUFA target action date until February 2023.
An end-of-week review of retina news and stories from October 29-November 3, 2022.

Scott Walter, MD, an investigator in the TENAYA and LUCERNE trials discusses the year 2 data for the treat-and-extend regimen of faricimab for the treatment of nAMD and DME.

Tom Ruggia, President and CEO of Samsara Vision, provides updates on the lead asset for late-state age-related macular degeneration: Smaller-incision, new generation, implantable miniature telescope (SING IMT).

A combination of posters, podium presentations, and instructional courses provided invaluable revelations to the ophthalmic community.
At AAO 2022, Justis Ehlers, MD, presented a talk entitled, "Defining the Fluid Problem in Neovascular AMD: To Dry or Not to Dry?"
Roger A. Goldberg, MD, MBA, discusses his 2022 AAO poster: "T&E-Based Personalized Treatment Interval Dynamics in the YOSEMITE/RHINE Trials of Faricimab in DME."
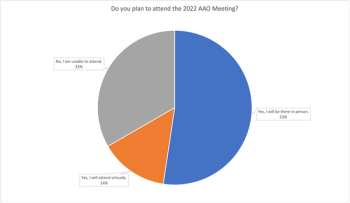
Results from our recent poll regarding AAO 2022 attendance indicate that most ophthalmologists and retina specialists plan to participate in the Annual Meeting in person in Chicago, Illinois.
Roche’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.

A poll for retina specialists regarding their attendance at the 2022 American Academy of Ophthalmology Meeting in Chicago, Illinois. The poll is now closed.

A synopsis of the findings presented at EURETINA 2022 for ophthalmologists and retina specialists.
Tunde Peto, MD, PhD, shares some ophthalmic initiatives she's excited about in light of EURETINA 2022.

Tunde Peto, MD, PhD, discussed two of her presentations at EURETINA 2022: "UK Biobank retinal imaging grading: methodology, baseline characteristics and findings for common ocular diseases" and "Retinal phenotyping of different variants of Alzheimer’s disease using ultra-widefield imaging."

Dara Conlon, Executive Vice President of EURETINA, and Prof. Anat Loewenstein, General Secretary of EURETINA, discuss some key features of this year's Congress.
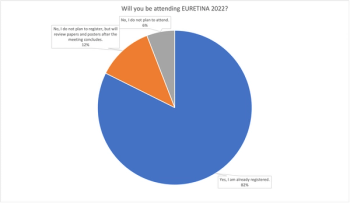
Results from our recent poll regarding EURETINA 2022 attendance indicate that most ophthalmologists and retina specialists plan to participate in the annual Congress in person in Hamburg, Germany.

A poll for retina specialists across the globe as the EURETINA 2022 meeting approaches. This poll is now closed.

Nancy Lurker, CEO of EyePoint Pharmaceuticals, shares the 12-month safety data from the DAVIO trial, investigating EYP-1901 for the treatment of wet age-related macular degeneration (AMD) and nonproliferative diabetic retinopathy.

Victor Gonzalez, MD, shares updates from the Phase 4 Paladin study regarding the safety and efficacy of fluocinolone in diabetic macular edema.

Claire Gelfman, PhD, Chief Scientific Officer of Foundation Fighting Blindness, supplies a brief overview of the foundation's aims and updates on retinal disease research.

At ASRS 2022, John Kitchens, MD, presented, “Personalized Treatment Interval Dosing Dynamics Over 2 Years in the Phase 3 YOSEMITE and RHINE Trials of Faricimab."

Published: September 19th 2021 | Updated: October 10th 2021

Published: June 28th 2021 | Updated: January 13th 2023

Published: October 24th 2021 | Updated: November 17th 2021

Published: May 18th 2022 | Updated: July 20th 2022
Published: August 5th 2021 | Updated: August 12th 2021
Published: April 27th 2022 | Updated: August 31st 2023