
The SAB additions include Usha Chakravarthy, MBBS, PhD; Allen Ho, MD, FACS, FASRS, and Frank Holz, MD, FEBO, FAVO.

The SAB additions include Usha Chakravarthy, MBBS, PhD; Allen Ho, MD, FACS, FASRS, and Frank Holz, MD, FEBO, FAVO.


The Phase 1/2 study includes a dose-escalation phase of the study featuring 3 cohorts each one receiving either a low, medium, or high dose of OCU410.

Modern Retina’s interview with Aracelis Torres, PhD, MPH sheds light on how real-world insights empower researchers to see trends and develop new strategies, sometimes even new products, to meet the treatment needs of patients.

Age-related macular degeneration continues to be a key development area for many pharmaceutical companies as the work to develop new treatment options and present data in the first few months of the year.

Research around the globe is working not only to develop treatments for AMD, but also to determine risk factors for the disease to help prevent future cases.

Nicole Bajic, MD, from the Cole Eye Institute at Cleveland Clinic shares her insights in preparing patients for the 2024 solar eclipse on April 8, 2024.

A total solar eclipse will cross 15 states on April 8, 2024. Durga Borkar, MD, MMCi shares how to communicate eye safety tips and prepare to treat anyone who does present with solar retinopathy.
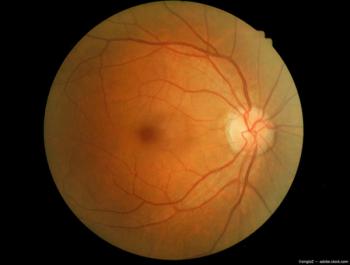
2024 is already bringing new research and insights to the forefront of retinal vein occlusion and providing new ways to consider retinal vascular health.
In this cohort of 3 adult patients, there was positive safety and efficacy data.

In addition to the primary safety and tolerability endpoints, the study also saw signals of biological response from EXN407
MCO-010 demonstrated a statistically significant improvement of best-corrected visual acuity (BCVA) at week 52.
Research estimates that the RVO market with see a compound annual growth rate of 11.9%.
After 3 loading doses, the patients were randomly assigned to receive EYP-1901 or continue to receive aflibercept at an 8-week dosing pattern for 32 weeks.

Advances in virtual reality and AI are helping those with AMD and low vision.

Ferrone’s role will be effective as of March 18, 2024.

The approval marks the first new steroid on the ophthalmic market in more than 15 years.

Anthony Mai, MD, shared results from using the miCOR 700 device, which is a new device from Zeiss, for phacoemulsification cataract surgery at the EnVision Summit 2024. He spoke with Peg Achenbach, OD, FAAO, Executive Director/Global Ambassador Strategies, Ophthalmology and Optometry at MJH Life sciences, on location in Puerto Rico to share more about his presentation.
These 3 participants have received their first aflibercept injection for the treatment of wet AMD.

Glenn Yiu, MD, PhD, was chosen for this grant. Yiu is a retinal specialist and clinician-scientist currently a professor of ophthalmology at the University of California, Davis.

Sydney M Crago, the editor of Modern Retina, talks with Arshad M Khanani, MD, MA, FASRS, about the expanded efficacy data from the GATHER 2 trial for geographic atrophy (GA).

This presentation will outline the safety and bioactivity of KSI-501ABC in patients with diabetic macular edema (DME).

The FDA previously granted Regenerative Medicine Advanced Therapy (RMAT) designation and orphan drug designation to ATSN-101 for the treatment of LCA1.

The commercial release of OcuLenz is planned for the first half of 2024. Ocutrx is collaborating with Medicare and insurance providers to ensure the headset's affordability and accessibility.
The kiosk is the first to offer self-service retinal imaging, empowering individuals to capture their own images.

This trial is a multi-center, open-label safety and tolerability study enrolling 30 patients to evaluate a low and high dose of AVD-104 with 3-month follow-up.

Updates include expected timelines for trials for the treatment of wet AMD, DME, GA, and inherited retinal diseases.

In a letter to shareholders, Ricciardi provided updates on Cognition’s 2024 pipeline and expected advances for several diseases.

This partnership will provide Greater Tampa Bay community with services for the treatment of uveitis and retina care.

If granted, this device would be assigned an official classification as a Class II device with special controls.