
The FDA previously granted Regenerative Medicine Advanced Therapy (RMAT) designation and orphan drug designation to ATSN-101 for the treatment of LCA1.

Beacon Therapeutics releases 12-Month Data from Phase 2 SKYLINE Trial of AGTC-501 for patients with X-linked retinitis pigmentosa
Ascidian Therapeutics announces first-ever IND for an RNA exon editor as FDA approves trial plan and fast tracks ACDN-01 in Stargardt Disease

The FDA previously granted Regenerative Medicine Advanced Therapy (RMAT) designation and orphan drug designation to ATSN-101 for the treatment of LCA1.
According to the company, all three patients treated for two years or more have remained free of symptoms and disease progression while taking gildeuretinol.

Updates include expected timelines for trials for the treatment of wet AMD, DME, GA, and inherited retinal diseases.

A specialist in the field of ophthalmology and gene therapy, Dr Girach’s appointment enhances SpliceBio’s leadership team as it accelerates lead program targeting Stargardt disease towards clinical development.
Catch up on a few of our top stories and ones you may have missed in 2023

During a multidisciplinary meeting with FDA, based on preliminary results from an ongoing Phase 1/2 study, the company received alignment on key points of the Phase 3 study design.

A genome topology map of human retina development lays the foundation for understanding diverse clinical phenotypes in simple and complex eye diseases.
According to the company, the Regenerative Medicine Advanced Therapy designation will help expedite the development of new regenerative medicines.

The partnership will further the effort to bring VGX-0111, a gene therapy treatment for AMD to patients and providers.

LambdaVision seeks to cure genetic blindness with a protein-based artificial retina. Harnessing microgravity in low-Earth orbit, the company collaborates with NASA and the ISS to perfect its manufacturing process.
Some animals have the built-in ability to regenerate retinal neurons by turning another retinal cell type called Müller glia into neurons. Researchers have been able to coax the human Müller glia into changing identity in the laboratory, which could serve as a potential source of new neurons to treat vision loss.
According to the company, ATSN-101 continues to demonstrate clinically meaningful improvements in vision at the highest dose and is well-tolerated 12 months post-treatment.

In the study, researchers changed the microenvironment in the eye in a way that enabled them to take stem cells from blood and turn them into retinal ganglion cells that were capable of migrating and surviving into the eye’s retina.
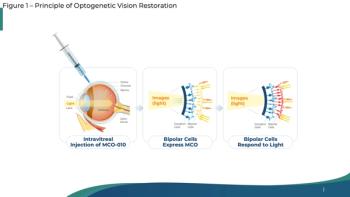
A mutation-agnostic optogenetic gene therapy is in development using multi-characteristic opsin (MCO) to sensitize the retinal bipolar cells to detect light.

ATSN-101, a gene therapy for GUCY2D-associated Leber congenital amaurosis, has demonstrated clinically meaningful improvements in vision at the highest dose with no drug-related serious adverse events 6 months post-treatment in ongoing Phase I/II clinical trial.

According to the company, OCU410ST uses an AAV delivery platform for the retinal delivery of the RORA (RAR Related Orphan Receptor A) gene.
Study finds that 1 in 3 children with sickle cell disease has retinopathy.

The company presented topline Phase I/II first-in-human results at the 127th AAO annual meeting in San Francisco, demonstrating significant improvement in visual field, in concordance with trended improvements in visual acuity and functional vision.
Researchers have delved into the possibility of cell-based therapy in ophthalmology. By targeting vitreoretinal diseases, they are tackling a range of vision-threatening disorders which often result in severe irreversible vision loss.

Researchers note their work reports a novel candidate gene for FEVR, which expands the spectrum of EMC1 variants and EMC1-related phenotypes, providing evidence for the prenatal diagnosis of candidate disease-causing EMC1 variants in FEVR.
The research will focus on the compound's impact on biomolecular condensates, which are implicated as a key driver of pathology in neurodegeneration and diseases impacting high metabolic organs. Because the retina is one of the highest energy consuming systems in the human body, deficits in energy supply can be catastrophic.
With a robust pipeline, new hope for Stargardt disease and retinitis pigmentosa may be on the horizon.

The use of VR mazes hold an intrinsic advantage compared with physical mazes in the ability to control conditions and test various patterns with reliability, participant safety, and repeatability.
According to the company, OPGx-LCA5 is designed to address vision loss due to Leber congenital amaurosis associated with mutations in the LCA5 gene, which causes one of the most severe early-onset retinal dystrophies.

According to the companies, the collaboration has the potential to benefit patients who have suffered from specific, chronic ocular diseases.