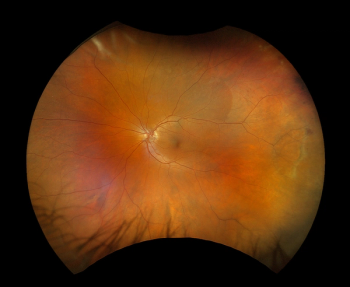
Optos announced the first ultra-widefield color rgb (red/green/blue) image. This new image modality is captured simultaneous to the optomap color rg image.

Optos announced the first ultra-widefield color rgb (red/green/blue) image. This new image modality is captured simultaneous to the optomap color rg image.

Results of the study, published in the Journal of Clinical Investigation, showed evidence that an experimental drug called 32-134D may prevent or slow vision loss in people with diabetes.

Oxurion NV has reached its enrollment target of 108 patients in its KALAHARI Phase 2, Part B clinical trial for diabetic macular edema (DME) and will likely continue to include additional participants in the trial due to high interest.

Alimera Sciences, Inc. has completed enrollment for its NEW DAY clinical trial (NCT04469595). Participants are patients diagnosed with diabetic macular edema (DME).

Curative Biotechnology Inc. has completed and filed a patent cooperation treaty (PCT) application with the U.S. Receiving Office.

AIV007 is a broad spectrum tyrosine kinase inhibitor, targeting the convergence of fibrosis, angiogenesis, and inflammation.

DIAMOND is a Phase 3, two-stage, double-masked, randomized, multi-center trial to assess the efficacy and safety of OCS-01 eye drops in DME patients. The primary objective of Stage 1 was to select the optimal dosing regimen.

Roger Goldberg, MD, MBA, spoke with Ophthalmology Times® to share insights about macular leakage area in the YOSEMITE and RHINE trials comparing faricimab to aflibercept.

Michael Ip, MD, highlighted the results of the 5 year follow-up of the original SCORE2 dataset from the NIH-sponsored clinical trial.

Allen Chiang, MD, discussed the assessment of geographic atrophy progression from the Phase 3 OAKS and DERBY trials with our team at the 2023 ARVO annual meeting.

Christine Kay, MD, discussed research for the treatment of GUCY2D associated Leber congenital amaurosis type 1 at this year's ARVO meeting held in New Orleans.

Caroline Baumal, MD, spoke with our onsite team at the 2023 ARVO annual meeting to share more about Apellis' presentations focusing on visual function, imaging and artificial intelligence.

Dierck Hillmann, PhD, was interviewed by our onsite team at the 2023 ARVO annual meeting where he shared research on holographic optical coherence tomography (OCT).

Theresa Heath, MD, MBA, spoke with our onsite team at this year's ARVO annual meeting in New Orleans where she shared insights regarding Non-viral gene therapy targeting ABCA4 retinopathies.

FDA has cleared Atsena Therapeutics' Investigational New Drug (IND) application for a Phase I/II clinical trial of ATSN-201 in patients with X-linked retinoschisis (XLRS).
Kiora Pharmaceuticals presented preliminary results for its investigational drug, KIO-301, a light-sensing small molecule designed to reactivate visual function of the eye in response to light.
Astellas Pharma has agreed to acquire Iveric Bio for a reported $5.9 billion (USD) with the goal of continuing progress in the innovation of ophthalmic therapeutics.

The presentations highlighted the Clearside Biomedical’s efforts in suprachoroidal delivery of agents to the back of the eye.

Kaushal Solanki, PhD, CEO and founder of Eyenuk, shared news about the company's EyeArt AI, which is cleared by the FDA to detect diabetic retinopathy.

Our onsite team spoke with Bruce Ksander, PhD, at the 2023 ARVO annual meeting, where he discussed his presentation about epigenetic reprogramming to reverse aging and restore function to retinal ganglion cells.

Lineage Cell Therapeutics Inc. presented results from the company's imaging analyses of structural changes and visual data from a Phase 1/2a clinical study of RG6501 (OpRegen), which is development as a potential treatment for geographic atrophy (GA).

Nanoscope Therapeutics Inc. presented key data at the ARVO 2023 annual meeting regarding their from its Phase 2b multicenter, randomized, double-masked, sham-controlled RESTORE clinical trial (NCT04945772) of MCO-010, which has received both orphan drug and fast track designations from the FDA.

Edmund Arthur, OD, PhD, FAAO, shared how retinal imaging can be an important tool for early detection of diabetic retinopathy in underserved communities.

Our team met with Jay Barth, MD, at the 2023 ARVO annual meeting, where he shared more about the work at Ascidian Therapeutics and how the company is developing a new method to administer gene therapy.

D-4517.2 is a novel precision nanomedicine that inhibits neovascularization by targeting activated microglia and hypertrophic retinal pigment cells, cells responsible for the increased vascularization associated with neovascular age-related macular degeneration (wet AMD) and diabetic macular edema (DME).

Belite Bio Inc. presented 18-month data from its ongoing two-year, open-label Phase 2 clinical study of LBS-008 (Tinlarebant), a novel oral therapy intended to reduce the accumulation of toxins in the eye that cause STGD1 and contribute to GA.
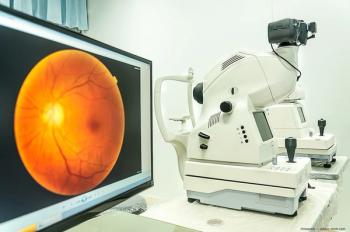
Genentech announced post-hoc data indicating treatment with faricimab-svoa (Vabysmo) led to greater and faster drying of retinal fluid with fewer injections for the treatments of wet AMD and DME, when compared to aflibercept.

Atsena Therapeutics announced positive 6-month safety and efficacy data from the ongoing Phase I/II clinical trial of ATSN-101, a gene therapy for the treatment of GUCY2D-associated Leber congenital amaurosis (LCA1).

Ophthalmology Times® spoke with Carl Danzig, MD, FASRS, at the 2023 ARVO annual meeting to learn more about the post hoc analysis for the GATHER trials and the new data on the correlation between vision loss and GA growth.

At the 2023 ARVO annual meeting in New Orleans, our on-site team caught up with T. Y. Alvin Liu, MD, to discuss predictive AI and its application in ophthalmology and screening of the eyes.