
Deborah Ferrington, PhD, spoke with on our on-site team at the 2023 ARVO annual meeting in New Orleans about her presentation on using human donor tissue to identify the mechanism responsible for the death of the retinal pigment epithelium (RPE).

Deborah Ferrington, PhD, spoke with on our on-site team at the 2023 ARVO annual meeting in New Orleans about her presentation on using human donor tissue to identify the mechanism responsible for the death of the retinal pigment epithelium (RPE).

Eyevensys reported data showing the efficacy and durability of EYS809, a dual-gene plasmid in development for the treatment of wet age-related macular degeneration (AMD).

At ARVO in New Orleans, Luxa Biotechnology shared an update on the progress of a Phase 1/2a clinical trial evaluating transplantation of RPESC-RPE-4W to treat dry age-related macular degeneration (AMD).

Amir H. Kashani, MD, PhD, joined us to share insights from his presentation at the 2023 ARVO meeting on optical coherence tomography and the assessment of cognitive impairment in Alzheimer's Disease and other related dementias.

Apellis Pharmaceuticals Inc. delivered an oral presentation at the ARVO Annual Meeting in New Orleans announcing their post hoc analyses from the Phase 3 OAKS and DERBY studies evaluating pegcetacoplan injection (Syfovre) for the treatment of geographic atrophy (GA).
Adverum Biotechnologies Inc. shared nonclinical data at ARVO 2023 in New Orleans that staggered, bilateral administration of Ixo-vec in NHPs was well tolerated with encouraging therapeutic activity and no signals of increased inflammation.

Clearside Biomedical plans to open enrollment for their Phase 2b clinical trial of CLS-AX this quarter. Topline results are expected by the Q3 of 2024.

New GA Won't Wait campaign partners Apellis Pharmaceuticals Inc. with actor Henry Winkler. The goal of this campaign is to raise awareness and educate older adults and their families about geographic atrophy (GA).
Vance Thompson, MD, joined David Hutton, Managing Editor, Ophthalmology Times®, to provide an overview of his presentation on pearls for cataract surgery at the Real World Ophthalmology meeting.
Jennifer Lim, MD, FARVO joined David Hutton, Managing Editor, Ophthalmology Times®, to discuss her presentation on keratoprosthesis combined with vitrectomy surgery given at this year's Vit-Buckle Society meeting.

Kala Pharmaceuticals KPI-012 receives FDA Fast Track designation as treatment for PCED with potential to treat retinitis pigmentosa and Stargardt disease.
J. Peter Campbell, MD, MPH, sat down with David Hutton, Managing Editor, Ophthalmology Times®, to discuss pediatric retina surgery and 3 key pearls to consider while performing the surgery.

According to National Eye Institute researchers, the variants generate malformed proteins that alter the stability of the membrane attack complex, which may drive a chronic inflammatory response in the retina.

No clinically relevant or dose-limiting adverse events were identified after repeated intravitreal injections.

According to the companies,Horus will increase Iluvien’s commercial presence with Nordic retinal specialists.
According to the company, clinically meaningful visual acuity gains were observed in several MCO-010 treated patients. The trial also demonstrated a favorable safety profile for MCO-010 with no serious or severe adverse events.

According to National Eye Institute researchers, the strategy may speed the delivery of therapeutic options relative to time it would take to develop gene therapies.
By crossing into the retina, researchers have found a new DHA supplement achieves what previous ones could not.

The Centers for Medicare and Medicaid Services accepted comments on the proposed rule and several organizations, including the American Academy of Ophthalmology, chimed in with comments and offered suggestions.

A team of researchers at Buck Institute, working with Google Health and Zuckerberg San Francisco General Hospital, are studying the eye as a window into human aging.

The partnership will offer specialty physician practices across retina and other specialties access to SamaCare's platform.

According to a team of researchers at UCLA, the “dormant” cone photoreceptors might actually continue to drive retinal activity for vision.

The FDA has approved enrolling pediatric patients in the ongoing OCU400 Phase 1/2 trial who have: 1) RP associated with NR2E3 and RHO mutations and 2) LCA associated with CEP290 gene mutations.

According to the company, UBX1325 monotherapy did not achieve non-inferiority through 24 weeks due, in part, to an unexpected 3.5 letter gain in the anti-VEGF control arm.

The company is advancing AAV-based gene therapy candidates toward IND studies on encouraging animal proof of concept data in Stargardt disease, X-linked retinoschisis and autosomal dominant optic atrophy.

According to researchers at the Max Planck Institute, the Kynurenine pathway is not only important for eye pigment formation, it also plays a role in maintaining retinal health.

Angela Carneiro, MD, PhD, discusses her position on a discussion titled Presence of Macular Neovascularization on OCTA is Predictive of Subsequent Exudation with David Hutton, Executive Editor, Ophthalmology Times®.

Antonio Campos, MD, PhD, discusses his position on a discussion titled Durability Associated with Multi-target Therapies is Superior to anti-VEGF Mono-target Therapy in AMD with David Hutton, Executive Editor, Ophthalmology Times.®

According to researchers, diabetes mellitus is caused by higher levels of blood glucose due to the lack of production of insulin by the body, resistance to insulin, or both.
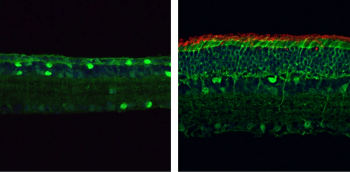
Researchers are using a new, highly versatile form of CRISPR-based genome editing with the potential to correct a wide variety of disease-causing genetic mutations.