
The IND approval will allow the company to initiate a Phase I/IIa clinical trial for its gene therapy treatment targeting wet Age-related Macular Degeneration (AMD) including Polypoidal Choroidal Vasculopathy (PCV).

The IND approval will allow the company to initiate a Phase I/IIa clinical trial for its gene therapy treatment targeting wet Age-related Macular Degeneration (AMD) including Polypoidal Choroidal Vasculopathy (PCV).


The UME module adds to the list of ophthalmic diseases available in the dataset.

At this year's ARVO meeting, Edmund Tsui, MD, presented a paper on the analysis of anterior chamber inflammation through automated quantitative assessment of swept-source anterior segment OCT images.

At this year's ARVO meeting, Roger Goldberg, MD, presented a paper on hard exudates and their resolution in patients with diabetic macular edema treated with either faricimab or 2 mg aflibercept.

At this year's ARVO meeting, Daniela Ferrara, MD, PhD, FASRS, presented a paper on predicting functional outcomes for different treatment durations of faricimab in diabetic macular edema.

At this year's ARVO meeting, Irene Santiago Tierno, MS, talked with Ophthalmology Times about the paper she presented on how abnormal subendothelial matrix exacerbates choroidal endothelial cell death in early age-related macular degeneration.

At this year's ARVO meeting in Seattle, Washington, the Eye Care Network spoke with Niranchana Manivannan, PhD. She spoke about the poster presentation, "Feasibility and clinical efficacy of an OCT B-scan of interest tool."

This year at ARVO, Emily Chew, MD, was awarded the Proctor Award Lecture. She spoke with the Eye Care Network about this lecture and what the award meant to her.

This year at ARVO, Anand Swaroop, PhD, was awarded the Friedenwald Award Lecture 2024. He spoke with the Eye Care Network about this lecture and what the award meant to him.

At this year's ARVO meeting in Seattle, Washington, the Eye Care Network spoke with Noel Brennan, MScOptom, PhD. The clinical research fellow at Johnson and Johnson shared highlights from his presentation on myopia control and predictive modeling.

At this year's ARVO meeting, the Eye Care Network spoke with Daniel Saben PhD, who is being awarded the Cogan Lecture at this year's event.

Elias Kahan speaks about his ARVO poster, "Contact specular microscopy reliably images the same location of the corneal epithelium."

At this year's ARVO meeting, Paul Kayne, PhD, gave insight on his paper titled "Mapping the melanocortin receptors in the human eye."

At this year's ARVO meeting, Deborah A Ferrington, MD, PhD, presented "Genotype-specific differences in mitochondrial function specific to the CFH Y402H risk allele associated with AMD."

At this year's ARVO meeting, Allen Ho, MD, presented a paper on presenting a paper on the 12 month results of a mutation agnostic optogenetic program for patients with severe vision loss from retinitis pigmentosa.

At this year's ARVO meeting, Dolly Chang, MD, PhD, presented data on the early fluid reduction in patients with diabetic macular edema, and how that correlates with 1-year outcome with a deep learning-based retina segmentation tool using the Phase 2 BOULEVARD study.

At this year's ARVO meeting, SriniVas R Sadda, MD, presented "Post Hoc Analysis of a Phase 3 Trial on SB15 (Proposed Aflibercept Biosimilar): Assessment on Pre-to-Post Switching Efficacy and Safety in Neovascular Age-related Macular Degeneration."

John Sheppard, MD, MSc, FACs, sat down to discuss post-hoc analysis of 2 FDA registration trials for perfluorohexyloctane, GOBI and MOJAVE, at this year's ARVO meeting held in Seattle, Washington, from May 5 to May 9.

At the ARVO meeting in Seattle, Washington, the Eye Care Network spoke with Giulia Corradetti, MD about AMD.

Barry Kuppermann, MD, PhD, director of the Gavin Herbert Eye Institute at the University of California Irvine, speaks about his ARVO poster presentation

Cynthia Roberts, PhD, sat down to discuss a 5-year prospective study to compare ocular stiffness parameters in, diabetes with retinopathy, diabetes without retinopathy, and normal subjects at this year's ARVO meeting held in Seattle, Washington, from May 5 to May 9.

At this year's ARVO meeting, Qinqin Zhang, PhD, presented a poster titled "A unified deep learning model for geographic atrophy segmentation: Adaptable to SS-OCT and SD-OCT data with multiple scan patterns." At the conference she gave Ophthalmology Times an overview.

At this year's ARVO meeting, Paolo Silva, MD, presented data on Protocol AA on behalf of the DRCR Retina Network and the effect of diabetic retinopathy lesion location and severity on the risk for progression in the long term.

At this year's ARVO meeting, Ash Abbey, MD, Director of clinical research at Texas Retina Associates, presented 36-month data from the GALE study of pegcetacoplan for the treatment of dry age-related macular degeneration specifically geographic atrophy.
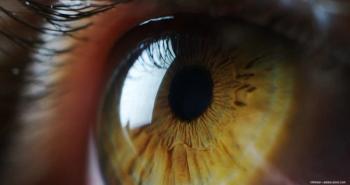
Kamuvudine-8, also known as K8, is administered to the back of the eye via a sustained released intravitreal implant.


The collaboration will bring automated, AI-powered OCT segmentation to MICRON Software Suite.

The 2 organizations to receive initial gifts will be Sight for Life and Eye Corps.

Stargardt disease is the most common inherited retinal dystrophy (causing blurring or loss of central vision) in both adults and children.