AMD
Latest News
Video Series

Latest Videos
Podcasts
CME Content
More News

8 things to know about aflibercept 8 mg for retinal vascular disease
A trio of retina specialists recently reviewed the clinical benefits of aflibercept 8 mg, including its extended dosing intervals, improved patient satisfaction, and enhanced treatment outcomes for various conditions.

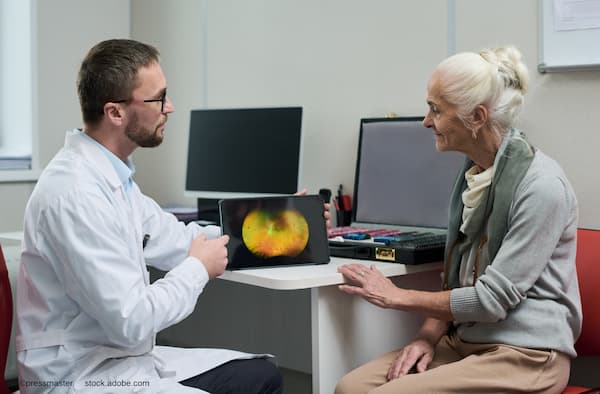
Knowing what’s on the market for AMD and GA aids in preserving vision.
The novel system converts light into electrical signals to stimulate retinal cells.

The FDA noted in the letter that it is unable to approve the application for ONS-5010/LYTENAVA (bevacizumab-vikg) in its current form for the treatment of wet AMD.

Remote monitoring of age-related macular degeneration enhances patient care, reduces treatment burden, and leverages AI for personalized management.

New treatments show promise in preventing fibrosis in neovascular AMD, addressing a critical need for improved visual outcomes in patients.

Anti-VEGF innovations in retinal disease: From molecules to medicine
Emerging anti-VEGF agents offer enhanced durability and anatomic outcomes in retinal disease.

New research highlights iron dysregulation's role in dry AMD, suggesting transferrin as a promising treatment to slow disease progression.

ONL Therapeutics randomly assigns first patient in phase 2 GALAXY trial evaluating xelafaslatide
Xelafaslatide (formerly ONL1204) is a small-molecule Fas inhibitor designed to protect key retinal cells from cell death that occurs across multiple retinal diseases and conditions such as geographic atrophy.


AAO 2025: Subretinal drusenoid deposits in Black and Hispanic patients with AMD
The investigators noted that this report is the first about subretinal drusenoid deposits in Black and Hispanic patients with age-related macular degeneration.

MacuMira launches first Health Canada-approved device for AMD treatment
The newly approved technology delivers low-dose microcurrents through closed eyelids to stimulate retinal cells.

Luxa Biotechnology announces clinical trial results for treatment of AMD
Luxa Biotechnology reveals promising phase 1/2a trial results for RPESC-RPE therapy, showing safety and potential vision restoration in patients with dry AMD.

Character Biosciences enhances its leadership team and secures $93 million in Series B funding to advance treatments for degenerative eye diseases.

Alteogen received a positive CHMP opinion for EYLUXVI in July 2025.

Belite Bio completes its phase 3 trial for tinlarebant, a potential first treatment for Stargardt disease, with results expected in late 2025.

Optain Health secures $26 million in Series A funding to enhance AI-driven retinal disease detection and expand its technology.
New study examines soft drusen regression without atrophy and drusen ooze
Researchers conducted this study to determine how often and why spontaneous soft drusen regresses without atrophy in patients who had intermediate or atrophic age-related macular degeneration.

University of Houston researchers receive $3.6 million to examine retinal diseases
Researchers will investigate a gene in the eye that is crucial for normal vision, but can cause retinal diseases when mutated that often lead to blindness.

Study links AMD to higher cardiovascular disease mortality in high-risk patients
The presence of AMD predicted an increased risk of all-cause and CVD mortality in patients with a high risk of CVD, even in the early stages of AMD.

The acquisition includes the noninvasive Valeda PBM device for the treatment of early and intermediate dry age-related macular degeneration.

A study reveals that ophthalmic artery angioplasty improves vision and functionality in patients with late-stage age-related macular degeneration.


FDA issues a complete response letter to Outlook Therapeutics for ONS-5010, citing insufficient evidence of effectiveness for wet AMD treatment.

Advanz Pharma and Alvotech announced AVT06, a biosimilar to Eylea, was approved for neovascular age-related macular degeneration.