
The clinical trial is examining the efficacy of two doses of UBX1325 compared to every other month treatment with aflibercept through 24 weeks.

The clinical trial is examining the efficacy of two doses of UBX1325 compared to every other month treatment with aflibercept through 24 weeks.

LUNA trial will evaluate the 2x10^11 vg/eye (2E11) and a new lower 6x10^10 vg/eye (6E10) dose of Ixo-vec, with enhanced prophylactic steroid regimens in patients requiring frequent anti-VEGF injections. Interim data anticipated throughout 2023.

The American Academy of Ophthalmology annual meeting is coming to Chicago, Illinois, from September 30 to October 3, featuring Subspecialty Days and a virtual component.

Oral APX3330 was found to demonstrate favorable safety and tolerability profile in interim masked safety results, consistent with 11 prior trials of APX3330.

Regeneron announced that the primary endpoints were met in two pivotal trials investigating novel aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration.
The first stage of the study will evaluate the safety and relative pharmacodynamic effect of different doses of subcutaneously administered D-4517.2 compared to intravitreal injection of aflibercept, an approved therapy, in both wet AMD and DME patients up to 12 weeks.
According to the National Institutes of Health, the therapy was derived from the patient’s blood by converting blood cells to iPS cells which were then programmed to become retinal pigment epithelial cells, which were surgically implanted as a patch of tissue.
According to a presentation by Oculis at EURETINA, the dataset shows that OCS-01 eye drops were more effective than vehicle in reducing central macular thickness and improving visual acuity in patients with DME as per the pre-defined criteria for statistical superiority in the study protocol.
Researchers at the University of Bonn are evaluating a new imaging technique for the diagnosis of posterior uveitis.

According to data from Prevent Blindness, there were more than 26,000 sports-related eye injuries treated in the United States last year.
Researchers in Trinity’s School of Genetics and Microbiology developed a new gene therapy, ophNdi1, that shows promise for treating the dry form of age-related macular degeneration (AMD).

Gene therapy has partly restored the function of the retina’s cone receptors in two children who were born completely colorblind, reports a new study led by UCL researchers.
According to the company, results showed increased effects over time with pegcetacoplan, with treatment effect accelerated between months 18 and 24.

The deal will expand Alcon's footprint in the ophthalmic pharmaceutical space and is expected to add broader pharmaceutical R&D capabilities to existing commercial efforts.
APOE4 gene associated with Alzheimer’s disease risk was found to protect mice from glaucoma. Research team also prevented retinal ganglion cell death by blocking the APOE signaling pathway, pointing to a potential treatment strategy for glaucoma.

Researchers at the Medical University of Vienna are focusing on how the retina can be used as a prognostic marker. Analyses revealed that retinal layer thinning as a result of an MS relapse predicts the severity of future relapses and the likelihood of disability.
With the support of Harrington Discovery Institute at University Hospitals, an ophthalmic therapeutic dubbed KIO-301, initially developed by Richard Kramer, PhD, at the University of California, Berkeley (UCB), has successfully been granted approval to start a Phase 1b, first-in-human clinical trial.

Combined, the companies say they will create the largest eye bank, tissue recovery and ocular research center in the world.
The U.S. Food and Drug Administration has approved Coherus’ ranibizumab-eqrn (Cimerli) as an interchangeable biosimilar for all five indications of Lucentis.

Kodiak Sciences Inc. announced that its BEACON Phase 3 study of tarcocimab, its novel antibody biopolymer conjugate, met the primary endpoint of non-inferior change from baseline in visual acuity at week 24 compared to aflibercept in patients with macular edema due to retinal vein occlusion.
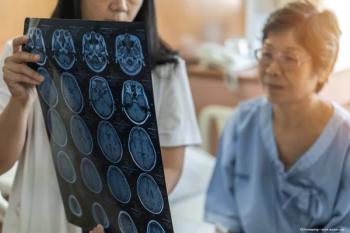
Investigators concluded that patients who are cognitively healthy with a high genetic risk of Alzheimer disease may exhibit changes in retinal tissue that correlate with brain changes.
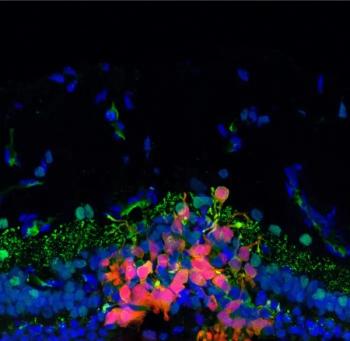
A multi-institutional effort led by researchers at the University of Pennsylvania is taking steps to develop an effective technique to regenerate photoreceptors cells and restore sight in patients with vision disorders.
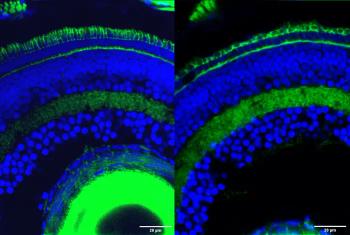
The findings could lead to a new understanding of unexplained causes of inherited retinal diseases.

A novel computational platform identifies top-performing viral vectors that could deliver gene therapies to the retina with maximum efficiency and precision.

According to EyePoint Pharmaceuticals, the clinical trial is reviewing EYP-1901, an investigational sustained delivery anti-vascular endothelial growth factor (anti-VEGF) treatment for wet age-related macular degeneration (wet AMD).
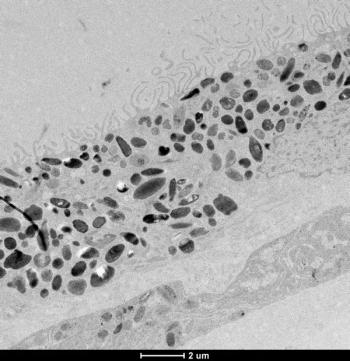
The discovery of molecular signatures of age-related macular degeneration will help with better diagnosis and treatment of this progressive eye disease.
A team of scientists, led by Andrzej Foik, PhD, of the International Center for Translational Eye Research, is working on new therapies that may slow vision loss in patients diagnosed with retinal degeneration.

Treatment reduces burden of care, providing another option for patients diagnosed with diabetic macular edema.
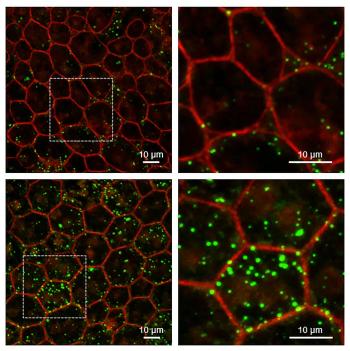
Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

Scientists at the Louisiana State University Health New Orleans Neuroscience Center of Excellence have developed a new, experimental human cell line from retinal pigment epithelial cells.