Geographic Atrophy
Latest News
CME Content


The company reported that statistically significant data was found supporting ANX007’s ability to provide vision loss protection in patients with geographic atrophy.

A Retina expert discusses approaches for treating geographic atrophy in patients who also have neovascular AMD, presenting specific patient cases and providing key insights for successfully managing such cases.

Carl D. Regillo, MD, FACS, FASRS presents a case study of a patient with bilateral geographic atrophy, discussing the decision-making process for determining which eye to treat first, considering factors such as the extent of atrophy and vision in each eye.

An expert retina specialist discusses the recent FDA-approved treatments for geographic atrophy (GA) that slow disease progression and highlights key factors to consider before starting treatment.

Carl D. Regillo, MD, FACS, FASRS, discusses geographic atrophy, the advanced stage of dry age-related macular degeneration, including its diagnosis, and progression.
Kamuvudine-8, also known as K8, is administered to the back of the eye via a sustained released intravitreal implant.
According to the company, OCU410 utilizes an AAV delivery platform for the retinal delivery of the RORA gene.

The Phase 1/2 study includes a dose-escalation phase of the study featuring 3 cohorts each one receiving either a low, medium, or high dose of OCU410.

Modern Retina’s interview with Aracelis Torres, PhD, MPH sheds light on how real-world insights empower researchers to see trends and develop new strategies, sometimes even new products, to meet the treatment needs of patients.

Since its US launch in September 2023, more than 40,000 avacincaptad pegol intravitreal solution vials have been distributed to physician practices.

The Phase 2 trial tested whether inhibiting microglia with minocycline might help slow GA expansion and its corresponding vision loss.
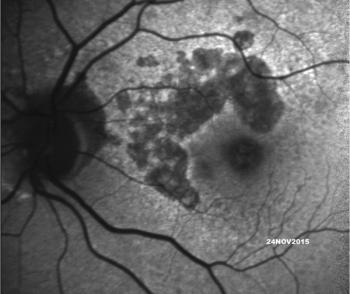
It is important that the ophthalmic community redouble our efforts in following patients with age-related macular degeneration (AMD) using optical coherence tomography (OCT) to find the early signs of geographic atrophy (GA).
The good news for patients is that GA is transitioning from an untreatable disease to a potentially treatable one with development of new therapies to reduce the growth rate, and use of anti-complement therapies have caused the growth rate of GA lesions to decrease.

OCU410 is a modifier gene therapy candidate being developed for geographic atrophy, an advanced stage of dry age related macular degeneration.
Early detection of the disease is key to optimal patient outcomes

According to the company, its proprietary non-viral gene therapy platform with minimally invasive delivery technology is providing long lasting gene expression and favorable distribution in the retina.

New treatments for geographic atrophy coming down the pike has the potential to change eye care providers' approach to identifying and managing the disease.
Trial will analyze efficacy, safety of AVD-104 versus avacincaptad pegol to treat GA

Sydney M Crago, the editor of Modern Retina, talks with Arshad M Khanani, MD, MA, FASRS, about the expanded efficacy data from the GATHER 2 trial for geographic atrophy (GA).

Allen Ho, MD, FACS, FASRS, will make a presentation at the meeting, to be held virtually on February 3.

The company’s ANX007 global pivotal program in geographic atrophy is expected to start in mid-2024. It is the first pivotal trial to use vision preservation as a primary outcome measure in GA.

In a letter to shareholders, Ricciardi provided updates on Cognition’s 2024 pipeline and expected advances for several diseases.
Catch up on a few of our top stories and ones you may have missed in 2023

Dr Charles Wykoff presents the results of a post hoc microperimetry analysis from the OAKS study demonstrating the effectiveness of pegcetacoplan in preserving visual function.