Patients treated every other month with the drug also showed a similar decrease in the rate of GA progression compared with sham treatment.

Patients treated every other month with the drug also showed a similar decrease in the rate of GA progression compared with sham treatment.

According to the study, pegcetacoplan injection reduced nonsubfoveal GA lesion growth by over 40% (monthly) in Year 3 compared to projected sham in the GALE extension study.
They believe that these phenotypic differences in GA should be considered in different ethnicities because they may have implications in research and in interventions to slow progression of GA.
The purpose of this investigation was 2-fold: to estimate the costs of treating GA with pegcetacoplan and to identify possible utility measures to compare treatments for GA.

This compound will be evaluated for the treatment of geographic atrophy (GA). The company shared that the first-in-human study is set to commence in 2024.

According to the company, AVD-104 is an engineered glycan (sialic acid) nanoparticle designed to target the self-pattern recognition receptors on overly activated retinal immune cells, specifically macrophages and microglia.
These continued studies also demonstrate a well-tolerated safety profile in a broad population of more than 1,200 patients.
Phase 2/3 SIGLEC trial are underway. Data shared at EURETINA 2023 shows continued safety with no drug-related adverse events thus far in cohorts 1, 2, 3, and 4.
The use of silicone-free syringes advised with these drops.

Visual acuity changes and conversion to neovascular AMD determined over 3 years in large patient cohort.
The authors of a case series say silicone oil droplets from silicone used to lubricate the McKesson syringes is the most likely cause of the droplets presumed to cause the floaters.
G6501 is a suspension of human allogeneic retinal pigmented epithelial (RPE) cells currently in development for the treatment of GA secondary to age-related macular degeneration (AMD).
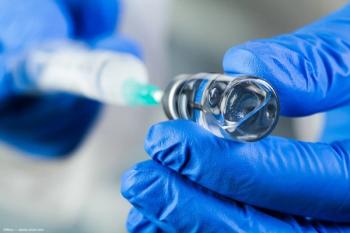
The company announced preliminary US net revenues of about $74 million for the injection, approved by the FDA earlier this year.

Kriya is developing a one-time gene therapy designed to block complement C3 and C5, which are clinically validated substrates targeted by FDA approved therapies, to delay the progression of geographic atrophy.
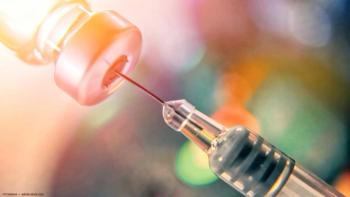
According to a news release, the J-code for SYFOVRE will become effective on October 1, 2023.

To educate patients on the disease, the company has enlisted the help of actor Eric Stonestreet and his mother, Jamey.

"Modern Family" actor and his mother, Jamey, are sharing their family’s story of GA and AMD to raise awareness of the disease.
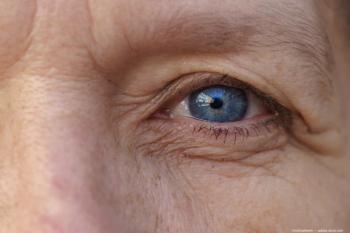
According to the company, GATHER-2 24-month results met the primary objective of reducing the rate of GA growth in patients treated with IZERVAY compared to sham.
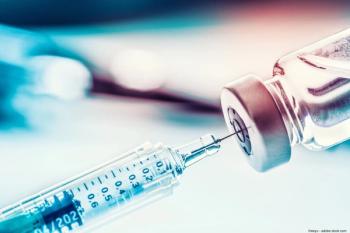
Garg reported the 30-month data showing that patients receiving pegcetacoplan experienced an increasing treatment effect compared with sham (ie, a decrease in GA lesion area over time).

The podcast by Cognition Therapeutics features a discussion with retinal specialists.

A retina specialist shares his presentation from the 2023 ASRS Annual Meeting, which describes a study comparing the efficacy of 2 FDA-approved treatments for geographic atrophy based on an anchored matching-adjusted indirect comparison of phase 3 trial findings.

We asked, "What research at ASRS 2023 do you find exciting or interesting?" Here's what Paul Hahn, MD, Kerrie Brady, BPharm, MBA, MS, and Michael Singer, MD had to say!

At the 2023 American Society of Retina Specialists meeting in Seattle, Washington, we asked some of the presenters, "What research here do you find exciting or interesting?" Here's what Diana Do, MD, Shawn Kavoussi, MD, and Durga Borkar, MD, MMCi had to say!

At the 2023 American Society of Retina Specialists meeting in Seattle, Washington, we asked some of the presenters, "What research here do you find exciting or interesting?" Here's what Ursula Schmidt-Erfurth, MD, Veena Raiji, MD, and Dante Pieramici, MD had to say!
According to the Keck School of Medicine of USC, the $12.4 million from the California Institute for Regenerative Medicine is the latest round of support for USC researcher Mark Humayun and a milestone in the development of a stem cell patch to treat advanced dry age-related macular degeneration.