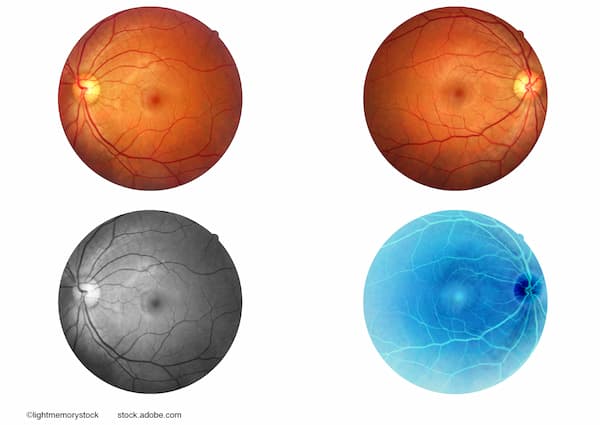
Geographic Atrophy
Latest News
Latest Videos

CME Content
More News

The Investigational New Drug (IND) application from Complement Therapeutics for CTx001 was previously approved by the FDA in October 2025.

Knowing what’s on the market for AMD and GA aids in preserving vision.
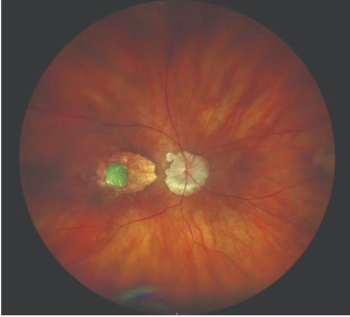
The novel system converts light into electrical signals to stimulate retinal cells.


This approach helps distinguish diseases associated with macular atrophy.

Atrophy Advisor aids physicians in personalizing treatment for geographic atrophy in age-related macular degeneration, enhancing patient care and outcomes.

Lock in patient adherence with discussion of study results.
Results showed reduction in geographic atrophy progression and improved visual acuity.

Xelafaslatide is a small-molecule Fas inhibitor designed to protect key retinal cells, including photoreceptors, from cell death that occurs across multiple retinal diseases and conditions.

New research highlights iron dysregulation's role in dry AMD, suggesting transferrin as a promising treatment to slow disease progression.

K8 is a member of a new class of inflammasome-inhibiting drugs called kamuvudines.

Geographic atrophy management evolves with proactive imaging, personalized therapies, and real-world insights, enhancing patient care and treatment outcomes.

To help with education and awareness, Prevent Blindness is providing free, expert-approved educational resources on GA.

In the study, authors investigated the natural history of GA lesion incidence rates and analyzed potential risk factors for faster incidence of GA lesions.

New five-year data reveals SYFOVRE significantly delays geographic atrophy progression, enhancing understanding of age-related macular degeneration treatment.

A study reveals that lesion characteristics in geographic atrophy significantly influence growth rates, highlighting the need for targeted treatment strategies.
Retina specialists share their perspectives on evolving anti-VEGF therapy, pipeline treatments, and strategies to enhance patient outcomes.

Fovea-sparing, multifocal, and bilateral lesions exhibited the fastest growth rates.


The newest research findings create "tangible benchmarks" for how earlier treatment can lead to better outcomes, said Dr. Kim.

Maximizing treatment based on GA location, lesion progression, and morphologic retinal changes.

David R. Lally, MD, discusses promising results from the ARCHER trial on ANX007 for age-related macular degeneration, emphasizing the need for vision-preserving treatments.

The company can now initiate the Opti-GAIN (Optimized Geographic Atrophy INterventional) phase 1/2 clinical trial.

Patient response focused on perceived vision-related quality of life outcomes, investigators said.

Character Biosciences enhances its leadership team and secures $93 million in Series B funding to advance treatments for degenerative eye diseases.