
According to the Nanoscope Therapeutics, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.

According to the Nanoscope Therapeutics, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.

A national poll suggests most parents overlook simple steps to protect children’s eyes; 1 in 7 parents say their child has not had a vision test in 2 years.

The ASRS "See for a Lifetime; See a Retina Specialist" initiative will spotlight early detection and outline the importance of seeing a retina specialist.

Pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.

At ASRS in New York City, Charles Wykoff, MD, presented a talk entitled, “Suprachoroidal Delivery of RGX-314 Gene Therapy for Diabetic Retinopathy: Phase II ALTITUDE Study.” The trial demonstrated large improvements for patients with diabetic macular edema (DME) and non-proliferative diabetic retinopathy (NPDR), with notable improvements according to the Diabetic Retinopathy Severity Scale (DRSS).

At ASRS in New York City, New York, David Eichenbaum, MD, presented “Efficacy, Durability, and Safety of Faricimab in Diabetic Macular Edema: 2-year Results on the Phase 3 YOSEMITE and RHINE Trials.”

At ASRS 2022, Raj K. Maturi, MD, presented a talk entitled, “UBX1325, A Novel Senolytic Therapy for Treatment- Experienced Patients With Chronic DME or Wet AMD: 24-Week Results of a Phase 1 Study.”

At ASRS in New York City, New York, Justis Ehlers, MD, presented a talk entitled, “Higher Order OCT Feature Assessments of the Impact of Fluid Dynamics on Visual Acuity in Neovascular AMD in a Phase III Clinical Trial: The Importance of Outer Retinal Integrity.” Here he discusses the findings.
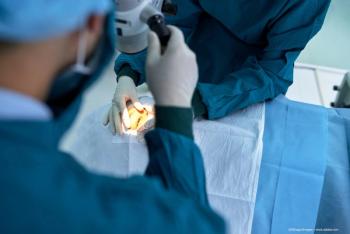
A team of investigators at the Agency for Science, Technology and Research in Singapore have developed a bio-functional thermogel, a type of synthetic polymer, to prevent retinal scarring caused by failed retinal detachment repair surgery.
A team of investigators from Johns Hopkins Medicine say they’ve discovered that levels of a specific protein appears to help accurately predict whether people with the wet form of age-related macular degeneration may need lifelong, frequent eye injections to preserve vision or if they can be safely weaned off the treatments.
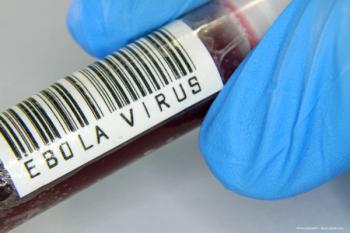
A team of investigators at Flinders University in Australia have found that a specific cell within the retina appears to be particularly good at housing Ebola and other viruses.

According to the company, the primary outcome of the MGT009 study is safety, and botaretigene sparoparvovec treatment was found to be generally safe and well-tolerated.

LumiThera Inc. noted that the trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

A team led by researchers from the University of Geneva has identified a molecular mechanism that causes degeneration of the eye’s photoreceptors, which can lead to blindness.

The U.S. Centers for Medicare and Medicaid Services code is effective July 1 and will enhance access to Xipere, the only therapy available in the United States for suprachoroidal use in the treatment of macular edema associated with uveitis.

A study by Orbis International found that children with myopia experienced significantly higher levels of depression and anxiety than their peers without vision impairment.
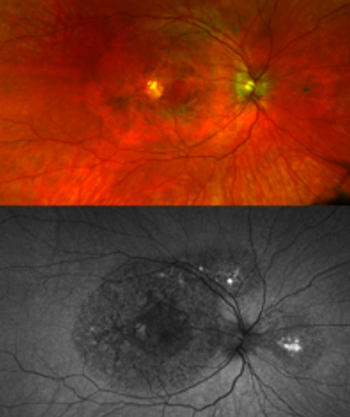
Genetic and clinical research reveals new type of macular dystrophy, a cause of central vision loss.

The study assesses retinal blood biomarkers using a new prototype OCT, aiming to measure retinal biomarkers such as blood flow volume, average velocity, and vessel diameter with a new prototype.
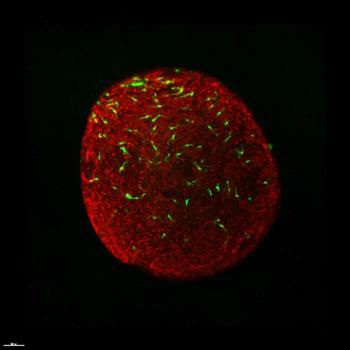
By co-culturing the differentiated microglia with retinal organoids, investigators found they may be able to recapitulate retinal development.

After 10 years, AREDS2 formula shows increased efficacy compared to original formula, benefit of eliminating beta-carotene.

With clearer imaging and enhanced resolution, the new OCT approach could improve medical diagnostic imaging.
According to the study by a team of researchers from the University of California Irvine and University of Southern California, treatment with Humanin G reduced protein levels of inflammation markers that become elevated in age-related macular degeneration.

While the DAZZLE trial failed to meet its primary endpoint, KSI-301 demonstrated good initial visual gains and anatomic effects as well as positive durability.

The study will investigate the safety, tolerability, pharmacokinetics, and efficacy of AM712 in subjects with neovascular age-related macular degeneration.


Biogen and Samsung Bioepis announce the launch of ranibizumab-nuna and detail when the ophthalmic biosimilar will be available for retina specialists.
A team of investigators has found that a comparison of ranibizumab and aflibercept showed both drugs safe and effective for treating diabetic macular edema.
According to Novartis, the approval is based on year 1 data from the Phase III KESTREL and KITE clinical trials investigating brolucizumab-dbll 6 mg vs aflibercept 2 mg in diabetic macular edema patients.
The company noted that pegcetacoplan, designed to regulate excessive activation of the complement cascade, part of the body’s immune system, which can lead to the onset and progression of many serious diseases, was granted Fast Track designation by the FDA for the treatment of geographic atrophy.
Outlook Therapeutics initially filed the BLA in March or the use of bevacizumab-vikg in the treatment of wet AMD. The company said it will re-submit a revised BLA by September.