
Center for Neuronal Longevity brings together the research power of the Keck School of Medicine of USC, USC Viterbi School of Engineering, and the USC School of Pharmacy to tackle neurodegenerative diseases of the eye and brain.

Center for Neuronal Longevity brings together the research power of the Keck School of Medicine of USC, USC Viterbi School of Engineering, and the USC School of Pharmacy to tackle neurodegenerative diseases of the eye and brain.
Work by a team of investigators is shedding light on the severity for gene variants and establishing outcome measures for therapeutic trials.

A University of California Davis study shows that small serving of the fruit increased protective pigments in the eye.
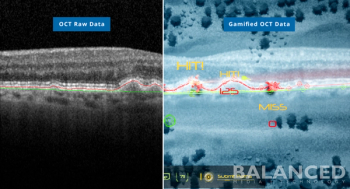
A partnership that includes BALANCED Media|Technology, the Retina Foundation of the Southwest and Southern Methodist University is seeking a patent for machine learning software for OCT images aids in identity progression and treatment options.

Nanoscope Therapeutics has received IND clearance from the FDA to begin a Phase 2 trial of its Multi-Characteristic Opsin ambient-light activatable optogenetic monotherapy to restore vision in Stargardt patients.
A team of investigators are working on a simple test that may someday identify those who can stop therapy.

Across four studies, about half of eligible faricimab patients were able to go 4 months between treatments, and approximately three-quarters could be treated every 3 months or longer. Two papers published in The Lancet highlight one-year results.

According to the company, biped.ai includes a comfortable and lightweight collar fitted with 3D cameras that continuously monitor a 170° field of view for the user detecting, tracking and predicting the trajectories of all surrounding elements a few seconds in advance.
Since joining the foundation in 2018, Menzo has been involved in the formation of the Retinal Degeneration Fund, the venture arm of Foundation Fighting Blindness.
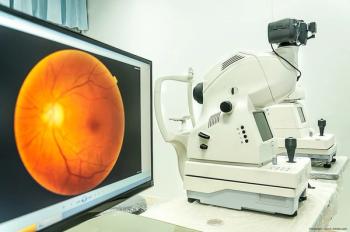
The difference between retina’s biological age and person’s real age linked to heightened death risk, with a team of Australian investigators finding that evidence suggests the microvasculature in the retina may be a reliable indicator of the overall health of the body’s circulatory system and the brain.

According to researchers, gaining a good understanding of what Musashi proteins do and how to manipulate their function could lead to the development of a universal therapy for blinding diseases.

ASCENT, REGENXBIO’s Phase III clinical trial conducted in partnership with AbbVie, is expected to enroll patients in the United States and Canada, with pivotal trials expected to support BLA submission for RGX-314 in 2024.

Researchers at the University of Virginia School of Medicine have made a discovery linking lupus, a potentially debilitating autoimmune disorder, and macular degeneration, a leading cause of blindness.

Under terms of the agreement, ImprimisRx to assume full responsibility for US sales and marketing activities for Dexycu.
Novartis has revealed the first interpretable results from year two (week 100) of the Phase III KESTREL study.

Jill Hopkins, MD, discusses results from phase 3a of the Merlin trial for brolucizumab as a treatment for wet AMD and provides updates on KESTREL and KITE trials for the treatment of diabetic macular edema.
Using its proprietary software, the company has been able to create models in a fraction of the time typically needed for fitting one.

AMA President Gerald E. Harmon, MD, said in a statement that President Joe Biden’s pandemic plan will make communities safer amid an evolving pandemic and confirmed cases of the Omicron variant.

According to the company, the board will support the advancement of late-stage ophthalmic assets Nyxol and APX3330.

The company’s re:Vive technology features 6 vision diagnostic exams, supported by 5 reimbursable CPT codes in 1 wearable solution.
The Children’s Vision Equity Alliance was created to improve access in children’s vision and eye health, working to advance equity in children’s vision and eye health through education, policies and partnerships.

Bausch + Lomb's Yolande Barnard shares an update on the FDA approval of XIPERE for the treatment of macular edema associated with uveitis.

ImprimisRx President John Saharek shares an update on a few of the company's latest products during AAO 2021.

Organizations are urging consumers to be aware of insurance policies that can limit their access to sight-saving procedures and treatments.

Two photographs are selected by an independent panel of five judges for this year’s honors.

Allergan developed VUITY, which it noted is the first and only FDA-approved eye drop to treat presbyopia.

The Cleveland Clinic named Rishi Singh, MD, president of the Cleveland Clinic Martin North and South hospitals in Martin County, Florida.

The Academy's annual meeting is set for November 12 to 15 in New Orleans.

Susvimo, previously called the Port Delivery System with ranibizumab, is a first-of-its-kind therapeutic approach for wet AMD and may help people with the disease maintain their vision with as few as two treatments per year.

Today marks World Sight Day, an annual day of awareness intended to focus global attention on blindness and vision impairment. Coordinated by the International Agency for the Prevention of Blindness, this year’s call is for everyone to “Love Your Eyes.”