
Jeff Cleland, PhD, CEO of Ashvattha, discusses safety data for an at-home subcutaneous anti-VEGF injection option in development for the treatment of wet AMD and DME.

Jeff Cleland, PhD, CEO of Ashvattha, discusses safety data for an at-home subcutaneous anti-VEGF injection option in development for the treatment of wet AMD and DME.

According to the company, the ReCLAIM-2 study of elamipretide demonstrates a correlation between ellipsoid zone dysfunction and vision.

Carlos Quezada Ruiz, MD, senior medical director at Genentech, discusses “Predicting optimal treatment regimen for patients with neovascular age-related macular degeneration using machine learning.”
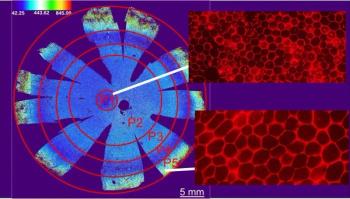
A discovery by investigators at the National Eye Institute sheds light on tissue targeted by age-related macular degeneration and other diseases.
Retina Technologies Inc. is working on a modular digital vision screening platform it says will provide vision testing for the early detection of eye disorders.

In a patient survey, 66% of respondents said AI plays a large role in their diagnosis and treatment and thought it was important.

The study found that if certain forms of nystagmus that are seen in patients, then there is a higher chance of poor binocular visual function, as well as higher interocular suppression.

As more and more practices embrace dark adaptation testing, AdaptDx technology has become a staple in primary eye care.

The American Academy of Ophthalmology applauded Congress for reaching the 290-consponsor milestone for the proposal. By reaching the two-thirds majority of bipartisan support in Congress, the bill is eligible for inclusion on the Consensus Calendar under new rules that were established in 2019.
According to the company, the trial did not demonstrate efficacy on the key clinical endpoints. As a result, Oxurion will now shift its focus to the Phase 2 development program for THR-149, which recently demonstrated a compelling safety and efficacy profile for the treatment of DME.

Dr. David Bingaman of Ora Clinical discusses today’s need to accelerate clinical studies and increase investments in retina and challenging disease states.
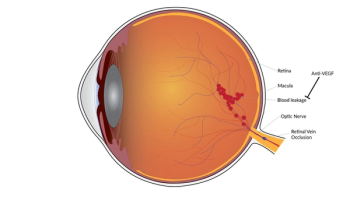
According to the study, many patients with retinal vein occlusion maintain visual acuity gains above baseline at five years, but they require long-term monitoring and treatment.
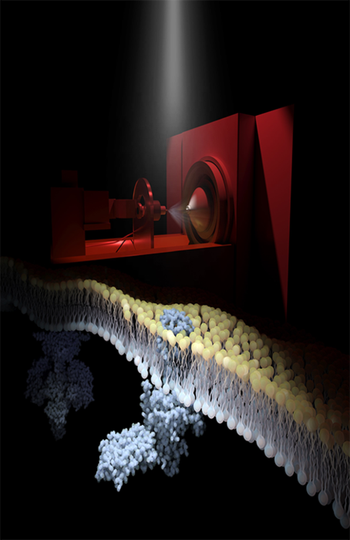
Impact of native lipids on rhodopsin signaling and regeneration opens door to GPCR drug discovery in native membrane environments.

Researchers at the University of Southern California have stimulated the retinas of blind mice using focused ultrasound technology.

According to the company, post-hoc analyses from Illuminate trial of sepofarsen demonstrate an encouraging efficacy signal when comparing active treatment and sham eyes to their corresponding contralateral eyes across multiple endpoints. The company plans to discuss findings with regulators in Q3.

The trial marks the first-ever in vivo delivery of an experimental CRISPR gene editing medicine to a pediatric patient, with the company on track to complete dosing of the pediatric mid-dose cohort in the first half of 2022.
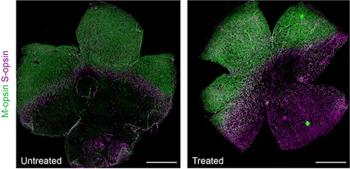
According to researchers at the University of California, Irvine, base editing may provide long-lasting retinal protection and prevent vision deterioration in patients with inherited retinal degeneration, specifically in Leber congenital amaurosis patients.

Investigators at the International Center for Materials Nanoarchitectonics have developed the first-ever artificial retinal device that increases the edge contrast between lighter and darker areas of an image, using ionic migration and interaction within solid.
Approval is based on year 1 data from the Phase III KESTREL and KITE trials investigating brolucizumab 6 mg versus aflibercept 2 mg in DME patients.
A University of Houston study found that minorities have fewer eye exams, higher instances of disease.

A team of investigators at the Okinawa Institute of Science and Technology Graduate University in Japan have identified a gene necessary for the survival of retinal ganglion cells—a class of neurons located in the retina that are critical for vision.

The company’s announcement marks first clinical trial in humans of Ocugen’s modifier gene therapy platform.
Horizon Therapeutics announces new data and a separate analysis will be presented at the American Academy of Neurology 2022 Annual Meeting, showing that UPLIZNA reduced disease activity associated with neuromyelitis optica spectrum disorder (NMOSD) over 3 years.
Bausch + Lomb and Clearside Biomedical Inc. are rolling out the new therapeutic, approved by the FDA for suprachoroidal use for the treatment of macular edema associated with uveitis.

If the Biologics License Application is approved by the FDA, the company could receive 12 years of marketing exclusivity for an FDA-approved alternative for the most frequently used anti-VEGF treatment in wet AMD patients in the United States.
The company notes that its clinical trial of the light delivery system meets the primary efficacy endpoint and can offer hope to patients with dry AMD who are experiencing vision loss and currently have limited treatment options.
According to a team of investigators at University of California, Berkeley, tests of the drug Antabuse could prove the role of hyperactive retinal cells in blindness, potentially leading to better therapies.

Researchers from the Department of Ophthalmology at the University Hospital Bonn and Deutsches Zentrum für Neurodegenerative Erkrankungen suggest that assessments of the eye’s retina could help to detect a loss of brain substance, ie “brain atrophy.” The findings are based on data from the Rhineland Study.

The clinical trial will evaluate the safety and tolerability of ADX-2191 in patients diagnosed with RP due to mutations of the rhodopsin gene, including the P23H gene mutation.

ProQR's Phase 2/3 trials center around its emerging therapy, ultevursen (previously named QR-421a), and are accepting patients with mutations in exon 13 of the USH2A gene.