
No treatment is currently available for the early stages of AMD, but some retrospective studies have reported an association between metformin and reduced risk of AMD.

Lynda Charters Enoch started her early “eye life” at the Schepens Eye Research Institute, Boston, which ultimately culminated in her current position as an Editor of Ophthalmology Times.

No treatment is currently available for the early stages of AMD, but some retrospective studies have reported an association between metformin and reduced risk of AMD.

Investigators concluded that both formulations of AR-1105 were well-tolerated and provided clinically meaningful and sustained visual improvements in vision and decreased retinal thickening despite that some patients had difficult-to-treat BRVO or CRVO with longstanding edema.
When dietary patterns were adjusted to include a Mediterranean diet, dietary nitrate intake was not associated with AMD progression independently.
The investigators set out to determine how well ophthalmologists actually are represented when individuals conduct a Google search compared with their proportion in each U.S. county.

The use of heavy silicone oil tamponade in secondary surgery for persistent full-thickness macular holes is a safe and efficient surgical method. Best-corrected visual acuity and minimum linear diameter before surgery may be indicators for anatomical success.

ROP is thought to be a two-stage disorder that begins with the arrested development of the retinal vessels in preterm babies when exposed to high oxygen levels at birth.
Spanish investigators found that local resection may offer better visual results and eye sparing without compromising local tumor control and survival.

In a multicenter phase 1/2 safety study conducted in the US and UK, researchers conducted a dose-escalation trial of AAV5-RPGR that included low, intermediate, and high doses of the gene therapy administered to 3, 4, and 3 adults, respectively.


Investigators examining end-stage RP witness the partial visual recovery in 2 patients.

Intravitreally injected anti-VEGF drugs are undergoing investigation as researchers look for new treatment options.

David Eichenbaum, MD, FASRS, shared difficult cases of diabetic macular edema (DME) and age-related macular degeneration (AMD) and insights into patient care.

In both patients and controls, investigators measured retinal nerve fiber layer thickness, foveal avascular zone area, and vessel density in the superficial capillary plexus, deep capillary plexus, and radial peripapillary capillary plexus.

In a real-world setting, eyes with AMD with a relatively good initial best-corrected visual acuity and no fibrovascular pigment epithelial detachment sustained or improved BCVA after anti-VEGF therapy within 3 years of follow-up.

The Sailing study was a 12-month, 18-center, randomized, double-masked, double-sham, parallel-controlled, phase 3 trial that was conducted in China and included eyes with center-involved DME that were randomly assigned to undergo either laser photocoagulation followed by as-needed sham intravitreal injections or sham laser photocoagulation followed by as-needed 0.5 mg intravitreal injections of conbercept.
In this retrospective, non-comparative clinical study, the investigators evaluated the preoperative, intraoperative, and postoperative data of the 225 study patients (295 eyes; mean patient age, 56.1 years) collected from several European centers.
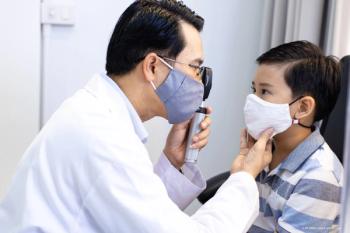
According to a study, health disparities impact the rates of pediatric ophthalmology visits.

In this cross-sectional study, investigators used OCTA to identify possible associations between body mass index and the waist-to-hip ratio with macular vessel density and foveal avascular zone in healthy Chinese adults.
The company noted that testing of a commercial supply of the implants, which included exposing them to multiple punctures with a needle, found they did not meet their standards. The company has notified the FDA, and is working with the agency on the recall process.

Investigators conducted a longitudinal study of the relationship between smoking and smoking intensity and the rate with which the retinal nerve fiber layer becomes thinner in patients with primary open-angle glaucoma.

The investigators suggested that the predicted metastasis-free survival for metastatic tumors may be worse than what they and other research groups have observed.

Anti-VEGF therapy for ROP has potential ocular and systemic advantages compared with laser but also noted that the systemic risks of anti-VEGF therapy in these infants need to be quantified.

The goal of World Sight Day, according to IAPB, is “to shine a light on blindness and vision impairment as a major, but solvable, public health issue."

Vision loss and depression are common conditions with major health implications. However, the mechanism of that association remains to be clarified.
Investigators reported that intravitreal steroid injections were the bases for more cases of endophthalmitis compared with other intravitreal injections.
The investigators undertook a study to determine the incidence of rhegmatogenous retinal detachments 1 year after cataract using the IRIS Registry data and to determine the demographic features, ocular comorbidities, and intraoperative factors associated with an increased risk of detachment development.
Patients with a unilateral rhegmatogenous retinal detachment frequently ask about the risk of developing an RRD in their fellow eye.

Those who used both traditional and electronic cigarettes reported severe to very severe ophthalmic symptoms.
The AVONELLE-X long-term extension study will continue to evaluate the efficacy, durability, and safety of faricimab in patients with neovascular AMD.

The data analysis showed that the macular thickness decreased across all time periods and in all macular regions for all tertiles.