Diabetic Macular Edema
Latest News

Phase 4 PALADIN results confirm durability of fluocinolone acetonide intravitreal implant for DME
Latest Videos

CME Content
More News
According to Novartis, the approval is based on year 1 data from the Phase III KESTREL and KITE clinical trials investigating brolucizumab-dbll 6 mg vs aflibercept 2 mg in diabetic macular edema patients.

Jeff Cleland, PhD, CEO of Ashvattha, discusses safety data for an at-home subcutaneous anti-VEGF injection option in development for the treatment of wet AMD and DME.

Professor Anat Loewenstein, MD, discusses data regarding the efficacy of faricimab at targeting both the VEGF and Ang2 pathways in patients with neovascular AMD and diabetic macular edema.
According to the company, the trial did not demonstrate efficacy on the key clinical endpoints. As a result, Oxurion will now shift its focus to the Phase 2 development program for THR-149, which recently demonstrated a compelling safety and efficacy profile for the treatment of DME.
The data presented at ARVO showed single subcutaneous doses of D-4517.2 were safe, well-tolerated in healthy subjects.
The trial will study the effect of the drug in treatment-naïve patients with diabetic macular edema (DME) who are members of underrepresented patient populations, ie, Black, Hispanic, Latin American, and Indigenous people.

Investigators found that patients with neovascular age-related macular degeneration in all countries included in the study lost vision as a result of the lockdown and reduced number of treatments during the COVID-19 pandemic.
Approval is based on year 1 data from the Phase III KESTREL and KITE trials investigating brolucizumab 6 mg versus aflibercept 2 mg in DME patients.
A University of Houston study found that minorities have fewer eye exams, higher instances of disease.
Bausch + Lomb and Clearside Biomedical Inc. are rolling out the new therapeutic, approved by the FDA for suprachoroidal use for the treatment of macular edema associated with uveitis.

Alimera Sciences, Inc. announces statistically significant improvements in best corrected visual acuity, central subfield thickness and treatment burden for patients with diabetic macular edema.
After 2 years, the improvements in vision and anatomy were sustained with extended dosing out to every 16 weeks in a high percentage of patients.

Joshua Mali, MD, talks about how the FDA approval of faricimab will change the treatment landscape for wet AMD and DME.

Genentech’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.

Approach shows potential as a promising second-line screening tool for patients with diabetes.
The improvement became evident during an average time of 6 months.

Consecutive intravitreal dexamethasone treatments may be beneficial for patients with DME patients who had undergone a previous vitrectomy.

In light of another unprecedented year filled with technological advancements and pivots as a result of the pandemic, Joshua Mali, MD, offers his top 5 predictions in ophthalmology for 2022.
The difference in visual acuities based on CST fluctuations in these patients with diabetic macular edema remained significant.

Current options, challenges in developing novel therapies.

Patients’ reluctance to visit a clinic during the COVID-19 pandemic is pushing retina specialists to optimize therapy.

Some studies have reported that retinal vascular permeability results in choroidal thickening, while others have reported choroidal thinning or no changes.
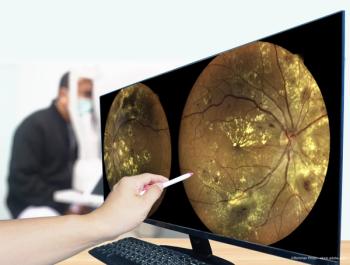
SD-OCT is providing reproducible, high-quality, registered images to assess the treatment response in macular disease.

Dr. Jorge Calzada shares his methods for treating diabetic macular edema with MicroPulse Laser and anti-VEGF injections.
Confronting issues unique to aging may lead to better outcomes.