Diabetic Retinopathy
Latest News

Researchers target screening rates for diabetic retinopathy among low-income minority patients

ASRS 2024: New data for Susvimo demonstrates sustained efficacy in DME, DR
Latest Videos

CME Content
More News
The results were presented at a symposium during the American Diabetes Association's 84th Scientific Sessions in Orlando, Florida, and mark the first large-scale trial specifically designed to investigate the effect of fenofibrate on eye outcomes in people with early diabetic retinopathy.
The mapping method revealed prominent microscopic abnormalities consistent with DR
Understanding how the intricate networks of blood vessels in the eye and brain are formed ultimately could inspire new treatments for conditions like diabetic retinopathy and stroke.

According to the company, GLOW2 is the second Phase 3 study of tarcocimab in diabetic retinopathy in which all patients on investigational therapy will receive tarcocimab on extended dosing intervals.
While it fell short of the primary endpoint of improvement of non-proliferative diabetic retinopathy (NPDR) of at least 2 Diabetic Retinopathy Severity Scale (DRSS) levels as of week 36, DURAVYU did demonstrate stable or improved disease severity with reduced rates of NPDR in 9 months.

At this year's ARVO meeting, Paolo Silva, MD, presented data on Protocol AA on behalf of the DRCR Retina Network and the effect of diabetic retinopathy lesion location and severity on the risk for progression in the long term.

The diagnostic screening technology is able to diagnose diabetic retinopathy by using 1 image taken of each eye with over 99% imageability.
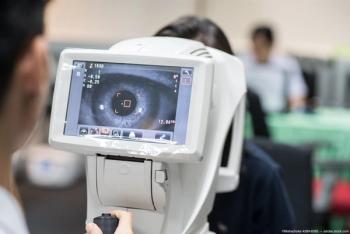
According to the company, 46.2% of patients demonstrated a 1- or 2-step improvement in the Diabetic Retinopathy Severity Scale (DRSS) at 40 weeks in the Axpaxli arm, compared to 0% in the control arm.
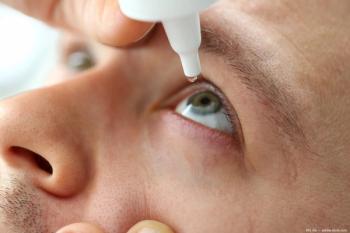
The trial evaluated nesvategrast (OTT166 5%) eye drops in patients with diabetic retinopathy.

Data will be shared in early 2024 regarding this potential treatment of diabetic retinopathy via eye drop.
EURETINA 2023 highlighted important DME and DR research and data from across the globe.

Researchers are addressing growing concern over vision loss in people taking semaglutideat the American Academy of Ophthalmology annual meeting in San Francisco.
The company made this decision after completing phase 2 trials of the drug.

DIAGNOS partners with Coordinated Accessible National Health Network to expand AI retinal screening across Canada
This effort will advance screening technology at Canada’s foremost specialist care provider in diabetes and endocrinology.

A new survey shows that 95% of adults at risk for certain retinal diseases know a little or nothing about them. Allen hopes her story will help raise awareness and encourage those at risk to regularly prioritize their eye health.
Priya Vakharia, MD, spoke with our team about her presentation at the Women in Ophthalmology Summer Symposium being held in Marco Island, Florida.

According to Regeneron, the approval was based on data in the PULSAR and PHOTON trials, in which the drug demonstrated clinically equivalent vision gains to aflibercept Injection 2 mg that were maintained with fewer injections.

Orbis International releases new research supporting artificial intelligence (AI) detection of diabetic retinopathy
According to Orbis International, the technology proves to be a practical solution in low-resource communities for tackling the leading cause of vision loss among working-age adults.

Kerrie Brady, Chief Executive Officer and President of OcuTerra Therapeutics shared updates from the company including the development of OTT166 at the 2023 ASRS annual meeting.

David Lally, MD, spoke with our team about the ZETA-1 phase 2 trial efficacy results for APX3330, a novel, oral ref-1 inhibitor for the treatment of diabetic retinopathy at the 2023 ASRS annual meeting.

Cindy Cai, MD, shared insights from her presentation from ASRS 2023 on "Health disparities in lapses in diabetic retinopathy care" with Sheryl stevenson, our Group Editorial Director.
Lim and colleagues conducted a retrospective study to establish the normalized NBFI using OCTA and test if that index is sufficiently sensitive for detecting early DR.
APX3330 achieved statistical significance in preventing clinically meaningful progression of diabetic retinopathy.

The DR:EAM trial will evaluate topically delivered OTT166 eye drops in adult patients with moderately severe to severe non-proliferative diabetic retinopathy (NPDR) or mild proliferative diabetic retinopathy (PDR) with minimal vision loss.