
KALAHARI study finds THR-149 to be safe, well-tolerated with preliminary efficacy as treatment for DME patients who respond suboptimally to anti-VEGF.

KALAHARI study finds THR-149 to be safe, well-tolerated with preliminary efficacy as treatment for DME patients who respond suboptimally to anti-VEGF.
Most patients (95%) with the PDS implanted did not need supplemental treatment before the refills, indicating the persistence and durability of the treatment.
During her talk at Angiogenesis, Dr. Loewenstein outlines how artificial intelligence could revolutionize diabetic retinopathy screening.
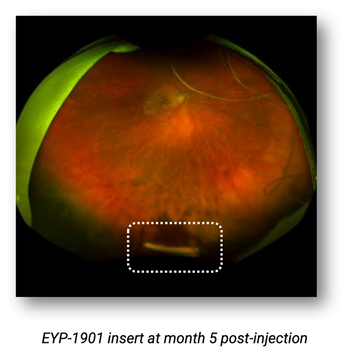
Previously treated patients showed significantly reduced treatment burden.
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.

In a presentation at the Bascom Palmer Eye Institute’s 19th annual Angiogenesis, Exudation, and Degeneration 2022 Virtual Edition, Glenn J. Jaffe, MD, noted that the analysis showed, for the first time, a decreased growth rate in the central foveal area by a therapeutic intervention when compared to sham treatment.
Dr. Nadia K. Waheed reviews the latest updates on the FOCUS trial, evaluating AAV-based viral vector GT005 for the treatment of geographic atrophy.

This study showed that an investigational subretinal implant, CPCB-RPE1, containing allogeneic human embryonic stem cell-derived RPE cells, was safe and well-tolerated by patients with dry AMD.
Dr. David S. Boyer describes how blocking Connexin-43 may improve the retinal vascular system function in patients with diabetes, potentially creating a future of oral medication for treatment of diabetic retinopathy and AMD.

A once-daily oral drug targets inflammatory processes and thus far has been found to be well tolerated in a phase 2 safety trial.

The meeting places a special emphasis on pharmacotherapies in the pipeline for the treatment of neovascular exudative diseases of the eye and how they will affect clinical practice and Medicare.

RGX-314 eyed by investigators as a therapeutic option for exudative AMD.

Preexisting neutralizing antibodies associated with the inflammatory changes.
Study results show that aflibercept-treated eyes are protected.
Annual Bascom Palmer Eye Institute program will spotlight latest advances in neovascular and exudative ocular disease over 2-day event, February 12-13.
Three sessions cover the range from clinical updates for imaging to emerging therapies for non-exudative AMD.

The program features an update on faricimab, plus diabetes, choroiditis, retinitis pigmentosa, and late-breaking reports.