
Case 1 highlights the potential for this treatment for patients with dry AMD with noted letter gains

Case 1 highlights the potential for this treatment for patients with dry AMD with noted letter gains

Robyn Guymer, MBBS, PhD, Deputy Director, Centre for Eye Research Australia, and Professor of Surgery (Ophthalmology), University of Melbourne, Australia, discussed the study findings at the 23rd annual Euretina Congress, Amsterdam.

Dr Marco Zarbin presents his talk from the 2023 ASRS annual meeting, where he explores the impact of disease activity criteria on drug durability estimations in clinical trials and includes a comparative analysis across multiple clinical trials in neovascular AMD (age-related macular degeneration).
According to a news release, the J-code for SYFOVRE will become effective on October 1, 2023.

"Modern Family" actor and his mother, Jamey, are sharing their family’s story of GA and AMD to raise awareness of the disease.
Few treatment options available for early stages of disease.
A panel of retina specialists review new and emerging treatments in neovascular AMD and DME, highlighting high-dose aflibercept and KSI-301. Which emerging treatments are you most interested in?

A panel of retina experts discussed the challenges presented in the management of DME. Which do you think is the biggest of these challenges?
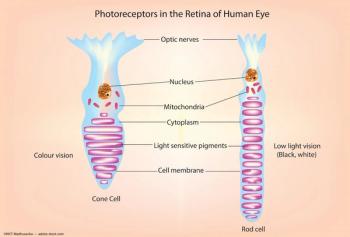
According to the Louisiana State University Health Sciences Center, elovanoids have been shown to restore the structure and integrity of damaged photoreceptor cells by repairing, remodeling and regenerating healthy cells.

A panel of retina specialists weighed in on the dosing intervals when using of faricimab for the treatment of neovascular AMD in the clinical practice setting. Do you agree with them?

A panel of esteemed retina specialists recently delved into innovative therapies for neovascular age-related macular degeneration and diabetic macular edema, focusing on personalized treatment, telemedicine, and the ongoing quest for advancements.

Novartis acquired Gyroscope Holdings Limited from Syncona in February 2022.

The podcast by Cognition Therapeutics features a discussion with retinal specialists.

A new survey shows that 95% of adults at risk for certain retinal diseases know a little or nothing about them. Allen hopes her story will help raise awareness and encourage those at risk to regularly prioritize their eye health.

The experimental gene therapy was administered as a treatment in part of a randomized, partially masked, controlled, phase 3 clinical study evaluating the efficacy and safety of an experimental therapy, ABBV-RGX-314, for wet AMD.

We asked, "What research at ASRS 2023 do you find exciting or interesting?" Here's what Paul Hahn, MD, Kerrie Brady, BPharm, MBA, MS, and Michael Singer, MD had to say!

Our team spoke with several researchers and industry professionals at the 2023 American Society of Retina Specialists meeting in Seattle, Washington. We asked them, "What research here do you find exciting or interesting?" Here's what Tarek Hassan, MD, Nancy Lurker, and J. Fernando Arevalo, MD, PhD, FACS, FASRS, had to say!

"What research at the 2023 ASRS meeting do you find exciting or interesting?" Here's what Aaron Lee, MD, Megan Baldwin PhD, and Carl Danzig, MD had to say!
According to the Keck School of Medicine of USC, the $12.4 million from the California Institute for Regenerative Medicine is the latest round of support for USC researcher Mark Humayun and a milestone in the development of a stem cell patch to treat advanced dry age-related macular degeneration.

According to Outlook Therapeutics, it is working with the FDA to address the agency’s issues.
Researchers successfully transplanted human microglia cells into a mouse retina to create a model that could be used to test new treatments for incurable eye diseases.

Adverum Biotechnologies, Inc. provided an update on the company’s ongoing Phase 2 LUNA trial evaluating ixoberogene soroparvovec (Ixo-vec) for the treatment of wet age-related macular degeneration (wet AMD).

The study met its primary efficacy endpoint for the neovascular age-related macular degeneration (nAMD) treatment.

According to Regeneron, visual gains and safety of aflibercept 8 mg remained consistent with the established profile of aflibercept 2 mg injection.

According to the company, the solution slowed the loss of photoreceptors and disease progression as early as six months.