Roche’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.

Roche’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.

Researchers found that the rate of visual decline increased significantly in patients with age-related macular degeneration during the COVID-19 lockdown who were followed over time.

LUNA trial will evaluate the 2x10^11 vg/eye (2E11) and a new lower 6x10^10 vg/eye (6E10) dose of Ixo-vec, with enhanced prophylactic steroid regimens in patients requiring frequent anti-VEGF injections. Interim data anticipated throughout 2023.

Automated quantitative fluid analyses are enabling personalised treatments, better patient outcomes

Regeneron announced that the primary endpoints were met in two pivotal trials investigating novel aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration.
According to the National Institutes of Health, the therapy was derived from the patient’s blood by converting blood cells to iPS cells which were then programmed to become retinal pigment epithelial cells, which were surgically implanted as a patch of tissue.
Researchers in Trinity’s School of Genetics and Microbiology developed a new gene therapy, ophNdi1, that shows promise for treating the dry form of age-related macular degeneration (AMD).
According to the company, results showed increased effects over time with pegcetacoplan, with treatment effect accelerated between months 18 and 24.
The U.S. Food and Drug Administration has approved Coherus’ ranibizumab-eqrn (Cimerli) as an interchangeable biosimilar for all five indications of Lucentis.

This year’s hybrid meeting will allow attendees to participate in person or virtually. Either way, this congress will offer a wealth of information to specialists covering all aspects of retinal diseases.

According to EyePoint Pharmaceuticals, the clinical trial is reviewing EYP-1901, an investigational sustained delivery anti-vascular endothelial growth factor (anti-VEGF) treatment for wet age-related macular degeneration (wet AMD).
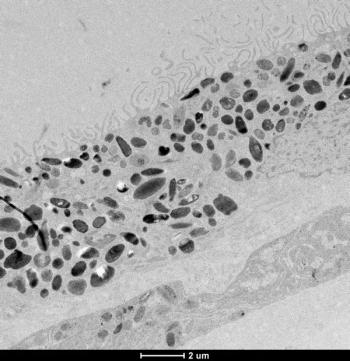
The discovery of molecular signatures of age-related macular degeneration will help with better diagnosis and treatment of this progressive eye disease.
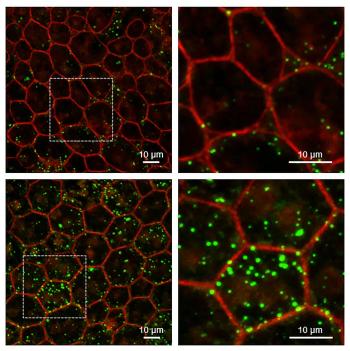
Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.

The ASRS "See for a Lifetime; See a Retina Specialist" initiative will spotlight early detection and outline the importance of seeing a retina specialist.

Pegcetacoplan is an investigational, targeted C3 therapy for the treatment of geographic atrophy secondary to age-related macular degeneration.

CAM encourages corneal healing in patients with ocular surface disease.
A team of investigators from Johns Hopkins Medicine say they’ve discovered that levels of a specific protein appears to help accurately predict whether people with the wet form of age-related macular degeneration may need lifelong, frequent eye injections to preserve vision or if they can be safely weaned off the treatments.

Rahul Khurana, MD, offers updates and details about the Aaviate study, which is investigating a possibly once-and-done gene therapy option for macular degeneration via suprachoroidal drug delivery.

LumiThera Inc. noted that the trial results demonstrated statistically significant improvement in the prespecified primary endpoint in BCVA at 13 months in the PBM treatment group over the sham-treatment group.

Study demonstrates results of pegcetacoplan as a geographic atrophy therapy.
Although off-label repackaged bevacizumab syringes appear to satisfy an urgent clinical and financial need for patients with a variety of retinal disorders, they are known to be associated with considerable public health concerns due to the risks posed to patients from compounding pharmacies’ lack of compliance with a variety of FDA requirements.

After 10 years, AREDS2 formula shows increased efficacy compared to original formula, benefit of eliminating beta-carotene.
According to the study by a team of researchers from the University of California Irvine and University of Southern California, treatment with Humanin G reduced protein levels of inflammation markers that become elevated in age-related macular degeneration.

The study will investigate the safety, tolerability, pharmacokinetics, and efficacy of AM712 in subjects with neovascular age-related macular degeneration.

Biogen and Samsung Bioepis announce the launch of ranibizumab-nuna and detail when the ophthalmic biosimilar will be available for retina specialists.