
As more and more practices embrace dark adaptation testing, AdaptDx technology has become a staple in primary eye care.

The company noted that pegcetacoplan, designed to regulate excessive activation of the complement cascade, part of the body’s immune system, which can lead to the onset and progression of many serious diseases, was granted Fast Track designation by the FDA for the treatment of geographic atrophy.
Additional information requested for BLA for ONS-5010, Outlook to resubmit by September

Results of Phase 2 ReCLAIM-2 study of elamipretide for dry AMD announced by Stealth BioTherapeutics

As more and more practices embrace dark adaptation testing, AdaptDx technology has become a staple in primary eye care.
Apellis revealed 18-month data for their investigational C3 therapy pegcetacoplan, unveiling continuous lesion growth reduction in eyes with geographic atrophy with monthly and every-other-month injections.
The data presented at ARVO showed single subcutaneous doses of D-4517.2 were safe, well-tolerated in healthy subjects.

According to the ALOFT study, patients demonstrate improved long-term vision in real-world setting after wet AMD conversion compared to current standard of care.

Jennifer I. Lim, MD, FARVO, FASRS, reviews the 2-year results of the YOSEMITE and RHINE trials, outlining the efficacy, durability, and safety of faricimab in diabetic macular edema.
The study found no association between treatment and the risk of chronic kidney disease or end-stage renal disease.
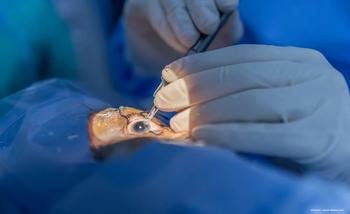
The axitinib intravitreal implant (OTX-TKI) is being evaluated to treat wet age-related macular degeneration in a phase 1b clinical trial.

A poll for retina specialists regarding their attendance at the 2022 Retina World Congress in Fort Lauderdale, Florida. This poll is now closed.

Apellis Pharmaceuticals releases results of their Geographic Atrophy Insights Survey (GAINS), revealing a large emotional toll and significant decrease in independence for patients with geographic atrophy.

A poll for retina specialists regarding their attendance at the Association for Research in Vision and Ophthalmology 2022 Meeting in Denver, Colorado. This poll is now closed.

Investigators found that patients with neovascular age-related macular degeneration in all countries included in the study lost vision as a result of the lockdown and reduced number of treatments during the COVID-19 pandemic.

If the Biologics License Application is approved by the FDA, the company could receive 12 years of marketing exclusivity for an FDA-approved alternative for the most frequently used anti-VEGF treatment in wet AMD patients in the United States.
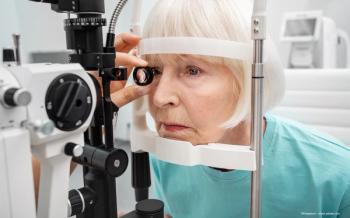
The company notes that its clinical trial of the light delivery system meets the primary efficacy endpoint and can offer hope to patients with dry AMD who are experiencing vision loss and currently have limited treatment options.
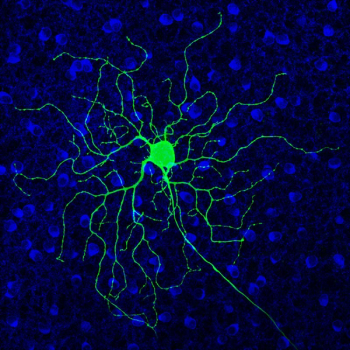
According to a team of investigators at University of California, Berkeley, tests of the drug Antabuse could prove the role of hyperactive retinal cells in blindness, potentially leading to better therapies.

The implant achieves impressive efficacy and reduces treatment burden.
Taiichi Hikichi, MD, counteracted the potential inflammatory effect of brolucizumab by combining its administration with a sub-Tenon’s capsule injection of triamcinolone acetonide.

Technology that allows consistent monitoring could be useful tools in 2022—and may be the key to tracking nAMD progression in fellow eyes among patients who have had intervals extended due to new technology.

According to the company, pegcetacoplan demonstrated continuous and clinically meaningful effects at month 18 in the studies, which also found that treatment effects in DERBY were comparable to OAKS during months 6 to 18. The combined 18-month data show the potential for improving treatment effects over time.
Firas M. Rahhal, MD, discusses concerns of using repackaged IV bevacizumab for the treatment of wet AMD.

Investigational pipeline provides promise for an unmet need.
The first FDA-approved bispecific antibody for the eye targets two leading causes of vision loss.
Reducing the treatment burden is a prime goal for emerging AMD therapies.

Presenters at the conference provided new evidence about detecting geography atrophy and wet and dry AMD early and predicting disease progression. Investigators are also focused on finding cures for inherited retinal diseases.

KSI-301, a therapy for patients with nAMD, did not meet the primary endpoint of showing non-inferior visual acuity gains compared to aflibercept given every eight weeks; however, it was safe and well-tolerated with no new or unexpected safety signals.

Kriya Therapeutics company recently took the wraps off an exclusive agreement with the Medical University of South Carolina (MUSC) Foundation for Research Development to license next generation complement-targeted gene therapies for the treatment of geographic atrophy and other ocular diseases.