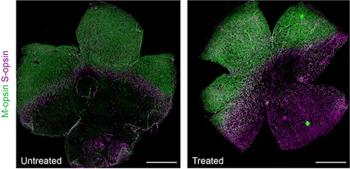
According to the company, post-hoc analyses from Illuminate trial of sepofarsen demonstrate an encouraging efficacy signal when comparing active treatment and sham eyes to their corresponding contralateral eyes across multiple endpoints. The company plans to discuss findings with regulators in Q3.