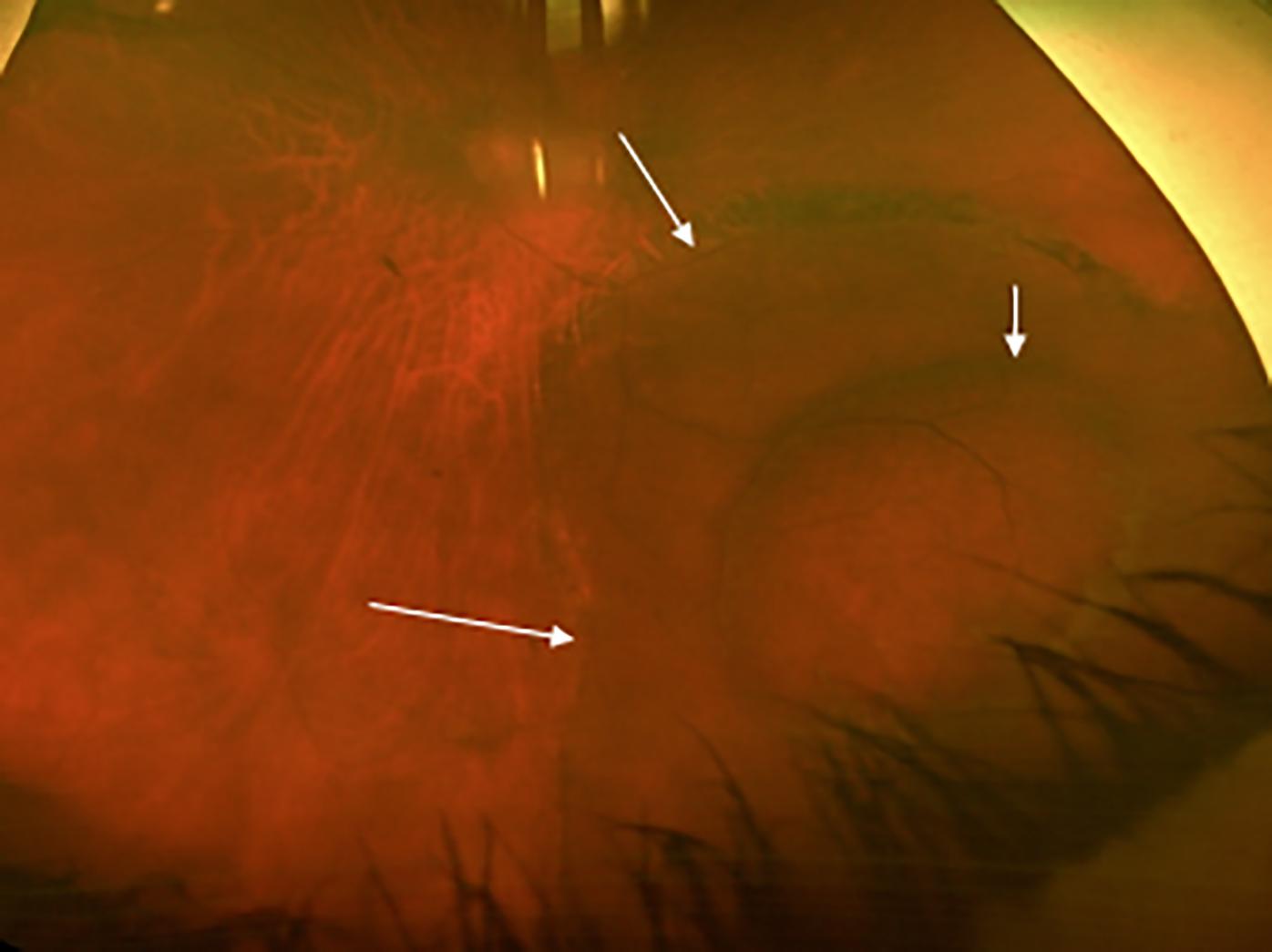
At ASRS in New York City, Charles Wykoff, MD, presented a talk entitled, “Suprachoroidal Delivery of RGX-314 Gene Therapy for Diabetic Retinopathy: Phase II ALTITUDE Study.” The trial demonstrated large improvements for patients with diabetic macular edema (DME) and non-proliferative diabetic retinopathy (NPDR), with notable improvements according to the Diabetic Retinopathy Severity Scale (DRSS).