
KALAHARI study finds THR-149 to be safe, well-tolerated with preliminary efficacy as treatment for DME patients who respond suboptimally to anti-VEGF.

KALAHARI study finds THR-149 to be safe, well-tolerated with preliminary efficacy as treatment for DME patients who respond suboptimally to anti-VEGF.
During her talk at Angiogenesis, Dr. Loewenstein outlines how artificial intelligence could revolutionize diabetic retinopathy screening.
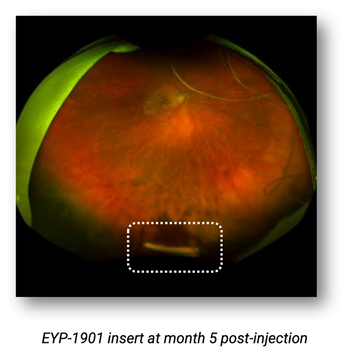
Previously treated patients showed significantly reduced treatment burden.
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.
Dr. Nadia K. Waheed reviews the latest updates on the FOCUS trial, evaluating AAV-based viral vector GT005 for the treatment of geographic atrophy.

This study showed that an investigational subretinal implant, CPCB-RPE1, containing allogeneic human embryonic stem cell-derived RPE cells, was safe and well-tolerated by patients with dry AMD.

A once-daily oral drug targets inflammatory processes and thus far has been found to be well tolerated in a phase 2 safety trial.
The latest invention from Mark S. Humayun, MD, PhD, brings hope to sufferers of age-related macular degeneration, a common type of blindness.

NGM621 is a monoclonal antibody product candidate engineered to potently inhibit complement C3 for the treatment of patients with geographic atrophy secondary to age-related macular degeneration.

Dr. Katherine Talcott discusses baseline factors—CST thickness, hemoglobin A1C, and baseline vision—and their effects on DME resolution.
Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.
The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.

A team of investigators has conducted ocular pharmacokinetic studies, including metabolism, providing valuable information for ocular drug development.

Center for Neuronal Longevity brings together the research power of the Keck School of Medicine of USC, USC Viterbi School of Engineering, and the USC School of Pharmacy to tackle neurodegenerative diseases of the eye and brain.
Investigators search for a link between transient ischemic attacks and changes in the retina.

A study finds acceptable outcomes, vision and microperimetry improvements at higher doses.
Work by a team of investigators is shedding light on the severity for gene variants and establishing outcome measures for therapeutic trials.

A University of California Davis study shows that small serving of the fruit increased protective pigments in the eye.
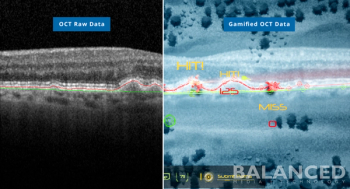
A partnership that includes BALANCED Media|Technology, the Retina Foundation of the Southwest and Southern Methodist University is seeking a patent for machine learning software for OCT images aids in identity progression and treatment options.

Joshua Mali, MD, talks about how the FDA approval of faricimab will change the treatment landscape for wet AMD and DME.

Genentech’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.

Nanoscope Therapeutics has received IND clearance from the FDA to begin a Phase 2 trial of its Multi-Characteristic Opsin ambient-light activatable optogenetic monotherapy to restore vision in Stargardt patients.
A team of investigators are working on a simple test that may someday identify those who can stop therapy.

Across four studies, about half of eligible faricimab patients were able to go 4 months between treatments, and approximately three-quarters could be treated every 3 months or longer. Two papers published in The Lancet highlight one-year results.

The historic Eye Institute rings in 60 years of leadership in eye care research, education and clinical practice.