
The Phase 3 integrated PEACHTREE and AZALEA study data revealed strong safety and efficacy for triamcinolone acetonide via suprachoirodal injection.

AAO applauds Congress for passage of key milestone for Improving Seniors' Timely Access to Care Act of 2021

The Phase 3 integrated PEACHTREE and AZALEA study data revealed strong safety and efficacy for triamcinolone acetonide via suprachoirodal injection.

Dr. Diana Do presents data on a novel patient-reported outcome instrument for patients with proliferative diabetic retinopathy with the hope of capturing the burden of the disease and treatment.
According to the company, the trial did not demonstrate efficacy on the key clinical endpoints. As a result, Oxurion will now shift its focus to the Phase 2 development program for THR-149, which recently demonstrated a compelling safety and efficacy profile for the treatment of DME.

Treatments include tinted spectacles and/or contact lenses, low-vision aids, atropine drops, and patching for amblyopia, as well as eye muscle surgery for the nystagmus or any anomalous head posture and associated strabismus.
According to an e-poster presented by Jagadesh C. Reddy, MD, and Harini Indusekar, BScOptom, at the ASCRS in Washington, D.C., patients who have pre-existing DR are less likely to achieve Snellen 20/40 or better vison after cataract surgery compared with the patients with diabetes but without DR.

The Association for Research in Vision and Ophthalmology’s 2022 annual meeting in Denver, Colorado, revealed a variety of advancements in technology, pivotal trials and clinical trial design.

Jennifer I. Lim, MD, FARVO, FASRS, reviews the 2-year results of the YOSEMITE and RHINE trials, outlining the efficacy, durability, and safety of faricimab in diabetic macular edema.

Investigators from the Karolinska Institutet and St. Erik Eye Hospital, Stockholm, Sweden, reported that they have developed a prognostic test, referred to as serUM, that they believe is a strong predictor of metastasis of uveal melanoma.

Eva Chamorro, PhD, MSc, points out that myopia control spectacle lenses affect the diurnal rhythms in the AL in young adult human and produced a small short-term increase in the AL that varies in intensity and time interval for each of the 3 studied lenses.
THR-687 is currently being evaluated in a phase 2 clinical trial in patients with DME. The first data from the dose-optimization study is expected to be released in the first half of 2022.

Mike Watson, Vice President of OraNet, unpacks some of the most pressing challenges to clinical research in today’s climate.

During a presentation at the Association for Research in Vision and Ophthalmology’s 2022 annual meeting in Denver, Yuichi Hori, MD, PhD, and colleagues found that taping the top border of a surgical mask to a clinicians’ skin reduces the potential for ocular surface damage resulting from expirations of air reaching the ocular surface during the COVID-19 pandemic.
The study found no association between treatment and the risk of chronic kidney disease or end-stage renal disease.

In a poster presented at the Association for Research in Vision and Ophthalmology’s 2022 annual meeting, Osama Ibrahim Hirayama, MD, and colleagues offered results that demonstrating that anisometropia and astigmatic error were greater among the patients with high myopia compared with the other groups. Compared with the subjects with no myopia, those with high myopia reported significantly more dryness, less photophobia, and less pain.

Dr. David Bingaman of Ora Clinical discusses today’s need to accelerate clinical studies and increase investments in retina and challenging disease states.
The axitinib intravitreal implant (OTX-TKI) is being evaluated to treat wet age-related macular degeneration in a phase 1b clinical trial.

Investigators from the New York University Grossman School of Medicine presented data at the ARVO 2022 annual meeting that concluded mapping of the relative cerebrovascular reactivity in the murine brain showed widespread brain changes resulting from the chronic IOP elevation and demonstrates vascular involvement in glaucoma both within and beyond the primary visual pathways.
At ARVO 2022, Jason S. Slakter, MD, presents data on the efficacy and safety of OPT-302 when combined with ranibizumab for polypoidal choroidal vasculopathy.

In a poster presented at the Association for Research in Vision and Ophthalmology’s 2022 annual meeting in Denver, Mamoru Ogawa, MD, noted that investigators have found that the choroidal and central corneal thicknesses increased over a very short period of time following intensive outdoor activity.

A poll for retina specialists regarding their attendance at the 2022 Retina World Congress in Fort Lauderdale, Florida. This poll is now closed.
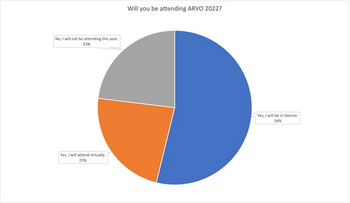
Results from our recent poll regarding ARVO 2022 attendance indicate that most ophthalmologists and retina specialists plan to participate in the Annual Meeting in person in Denver, Colorado.
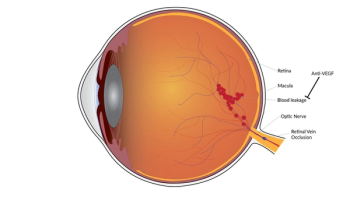
According to the study, many patients with retinal vein occlusion maintain visual acuity gains above baseline at five years, but they require long-term monitoring and treatment.
A variety of surgical advances were presented at ASCRS; here is a brief overview for retina specialists.

Apellis Pharmaceuticals releases results of their Geographic Atrophy Insights Survey (GAINS), revealing a large emotional toll and significant decrease in independence for patients with geographic atrophy.

Over the past 2 years, retina specialists and their patients have learned how AI may be a tool physicians can use to monitor patients for disease progression, how telemedicine might mean more than a mere video chat between patient and clinician, and how the tools of the 21st century were closer to real-world practice than anticipated.