Wet AMD
Latest News
Latest Videos

CME Content
More News
Taiichi Hikichi, MD, counteracted the potential inflammatory effect of brolucizumab by combining its administration with a sub-Tenon’s capsule injection of triamcinolone acetonide.
Firas M. Rahhal, MD, discusses concerns of using repackaged IV bevacizumab for the treatment of wet AMD.
The first FDA-approved bispecific antibody for the eye targets two leading causes of vision loss.
Reducing the treatment burden is a prime goal for emerging AMD therapies.

KSI-301, a therapy for patients with nAMD, did not meet the primary endpoint of showing non-inferior visual acuity gains compared to aflibercept given every eight weeks; however, it was safe and well-tolerated with no new or unexpected safety signals.
At Angiogenesis, Dr. David Brown presented the Phase 2 results of the CANDELA study for high dose aflibercept 8 milligram for wet AMD; here, he discusses those results.

Robert Bhisitkul, MD, PhD, provided 24-week clinical data from the Phase 1 study, demonstrating that patients with DME and wet AMD showed improved visual acuity through 24 weeks following a single dose of UBX1325.

A brief overview of findings presented at the 2022 Angiogenesis, Exudation and Degeneration meeting hosted by Bascom Palmer.
Most patients (95%) with the PDS implanted did not need supplemental treatment before the refills, indicating the persistence and durability of the treatment.
Previously treated patients showed significantly reduced treatment burden.
At Angiogenesis, Dr. SriniVas R. Sadda discusses how choriocapillaris may predict the rate of progression of atrophy.

According to the companies, a social media initiative will include donation to Prevent Blindness’ Sight-Saving Fund.

Genentech’s treatment of faricimab is the first and only FDA-approved medicine targeting two distinct pathways, angiopoietin (Ang)-2 and vascular endothelial growth factor (VEGF)-A, that often cause retinal diseases that may cause visual loss.
A team of investigators are working on a simple test that may someday identify those who can stop therapy.

Across four studies, about half of eligible faricimab patients were able to go 4 months between treatments, and approximately three-quarters could be treated every 3 months or longer. Two papers published in The Lancet highlight one-year results.

Firas Rahhal, MD, discusses Outlook Therapeutics’ Phase 3 pivotal NORSE TWO trial for ONS-5010.

ASCENT, REGENXBIO’s Phase III clinical trial conducted in partnership with AbbVie, is expected to enroll patients in the United States and Canada, with pivotal trials expected to support BLA submission for RGX-314 in 2024.

In light of another unprecedented year filled with technological advancements and pivots as a result of the pandemic, Joshua Mali, MD, offers his top 5 predictions in ophthalmology for 2022.

Jill Hopkins, MD, discusses results from phase 3a of the Merlin trial for brolucizumab as a treatment for wet AMD and provides updates on KESTREL and KITE trials for the treatment of diabetic macular edema.

Patients can get real-time disease monitoring with self-operated device.

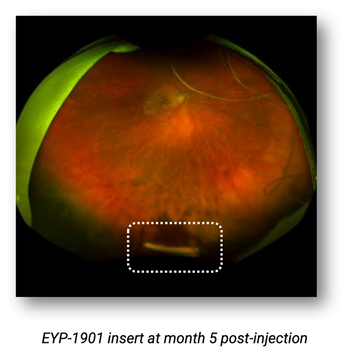
Jay Duker, MD, discusses EYP-1901, EyePoint Pharmaceuticals’ sustained-release anti-VEGF drug for the treatment of wet AMD.
Options facilitate increased durability for patients with retinal degeneration.

Carl Regillo, MD, takes us through the history of the port delivery system, from ideation to FDA approval.

Susvimo, previously called the Port Delivery System with ranibizumab, is a first-of-its-kind therapeutic approach for wet AMD and may help people with the disease maintain their vision with as few as two treatments per year.

During a presentation at the EURETINA 2021 Virtual Congress, Prof. Ben Burton detailed how dry AMD patients treated with photobiomodulation therapy have seen improvements in best-corrected visual acuity 9 months after treatment.