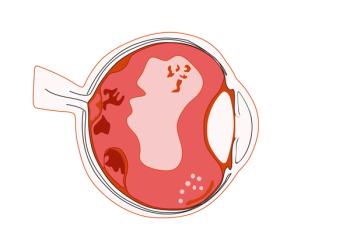
Wet AMD
Latest News
Latest Videos

CME Content
More News
Investigators corroborate the hypothesis that retinal ganglion cells with dendrites stratified in the off sublaminae could be damaged.

Amid pandemic, physicians and patients have embraced remote technology.
An updated PDS procedure reduces complications.

The not-for-profit vision and healthcare organization breaks ground on premier Technology Center for assistive technology for people with vision loss.
The company is hoping to commercialize the first FDA-approved ophthalmic formulation of bevacizumab-vikg for retinal disease. It expects to receive BLA approval from the FDA by mid-2022.

EYS809, a non-viral gene therapy sustained drug-delivery product that delivers anti-vascular endothelial growth factor to the eye, may replace the need for repeated intravitreal anti-VEGF injections and improve vision in patients diagnosed with wet age-related macular degeneration.
This novel intravitreal gene therapy may also hold potential for the treatment of diabetic macular edema.

Injections and implants continue to create further positive outcomes for patients.
Novel option demonstrates stable to improved visual acuity and retinal thickness

Minimizing clinic visits, maximizing use of imaging modalities are key
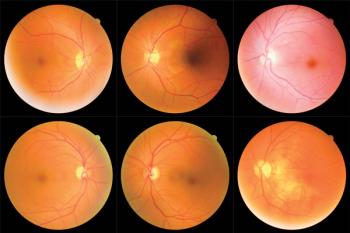
Rapid fluid identification may result in better visual outcomes
Specialist explains approaches for a variety of cases encountered during procedure
Anat Loewenstein, MD, provides an update on the latest developments in emerging therapies for the management of age-related macular degeneration (AMD) and wet AMD.

Joshua Mali, MD, discusses the three major risk factors for developing age-related macular degeneration (AMD).
Ian C. Han, MD, dives into the basics of age-related macular degeneration (AMD) and what patients need to know.

Rishi P. Singh, MD, highlights various treatment options available for the management of patients with age-related macular degeneration, as well as the status of potential therapies.

Analyses of IRIS Registry data capture a snapshot of patient visits and receipt of anti-VEGF injections.

Prospective randomized trial demonstrates clinical equivalence of biosimilar and reference ranibizumab