Confronting issues unique to aging may lead to better outcomes.

Confronting issues unique to aging may lead to better outcomes.

Nathan Steinle, MD, discusses top-line results of the OAKS and DERBY trials, testing the efficacy of pegcetacoplan for the treatment of geographic atrophy.
A team of investigators from the University of Michigan may have unlocked a potential new recipe for counteracting the impact that COVID-19 has on patients with underlying disease processes such as type 2 diabetes.

The Cleveland Clinic named Rishi Singh, MD, president of the Cleveland Clinic Martin North and South hospitals in Martin County, Florida.

Carl Regillo, MD, takes us through the history of the port delivery system, from ideation to FDA approval.

The Academy's annual meeting is set for November 12 to 15 in New Orleans.

The investigators reported the following most prominent cognitive deficits: processing speed, executive functioning, and phonemic fluency among others.
XIPERE, the first therapy utilizing the suprachoroidal space, demonstrates rapid efficacy, durable benefit, and favorable safety profile.

Susvimo, previously called the Port Delivery System with ranibizumab, is a first-of-its-kind therapeutic approach for wet AMD and may help people with the disease maintain their vision with as few as two treatments per year.

Dr. Victor Gonzalez discusses his presentation at ASRS, regarding 0.19mg fluocinolone acetonide implants in diabetic macular edema (DME) patients.

Nimesh Patel, MD, discusses his presentation entitled, "Scleral-Sutured Intraocular Lens Dislocations Secondary to Eyelet Fractures" at ASRS.

Luminopia One is one of few digital therapeutics to receive FDA approval via the de novo pathway, and it is the first for amblyopia, and for a neuro-visual disorder in general.

In his presentation at ASRS 2021, Dr. Michael Singer divulges the results of the phase 2 study of formulations used to decrease macular edema in patients with retinal vein occlusion.

Leo Kim, MD, PhD, discusses his presentation at ASRS 2021, entitled "Rho-kinase inhibition on an in vitro patient-derived model of proliferative vitreoretinopathy."
Investigators find that long-term ocular complications require further observation.

The development-stage biotechnology company Omega Therapeutics is teaming up with Stanford University to investigate therapies for ocular diseases via control of specific ocular disease genes.

On October 14, we celebrate World Sight Day by pledging to receive annual eye exams, preventing or slowing vision loss, and showing love for our eyes.

Today marks World Sight Day, an annual day of awareness intended to focus global attention on blindness and vision impairment. Coordinated by the International Agency for the Prevention of Blindness, this year’s call is for everyone to “Love Your Eyes.”

A review of studies, presentations and discussions at ASRS 2021 in San Antonio, Texas.

Ankur Shah, MD, discusses key highlights of his presentation titled "YUTIQ CALM: a real-world registry study of the fluocinolone acetonide intravitreal implant 0.18 mg in chronic non-infectious posterior uveitis," during ASRS 2021.

Paul Hallen discusses the Retina Industry Insights panel at the Ophthalmology Innovation Summit in San Antonio, Texas.

The FDA approved Ocular Therapeutix Inc.’s Supplemental New Drug Application (sNDA) that further extends Dextenza’s (dexamethasone ophthalmic insert) 0.4 mg indications, the company announced Monday.
During the American Society of Retina Specialists 2021 annual meeting, David Boyer, MD, told attendees that pegcetacoplan used to treat geographic atrophy proved to be well tolerated by patients in the phase 3 DERBY and OAKS studies.
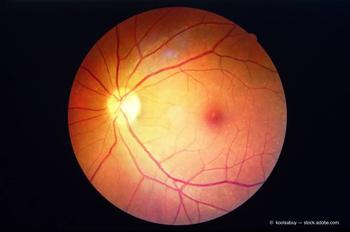
During a presentation at the American Society of Retina Specialists 2021 annual meeting in San Antonio, W. Lloyd Clark, MD, presented results of the PANORAMA Study. The focus of the study, Clark said, was to identify the factors that increase the risk of complications in patients with nonproliferative diabetic retinopathy to provide information for making decisions on treatment choices.

The EIDON Ultra-Widefield Lens module from iCare USA has received 510(k) approval from the FDA for distribution in the US.