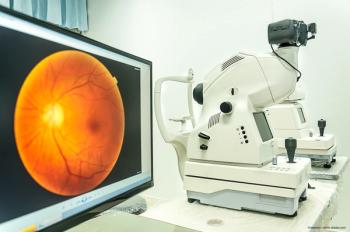
Option detects treatment-naive nonexudative macular neovascularization in eyes with dry age-related macular degeneration.

Option detects treatment-naive nonexudative macular neovascularization in eyes with dry age-related macular degeneration.
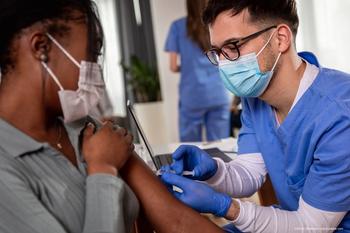
The American Academy of Ophthalmology issued a guidance this week requiring all in-person attendees at its annual meeting Nov. 12-15 in New Orleans to show proof of COVID-19 vaccination.
The American Society of Retina Specialists is gearing up for its 39th annual scientific meeting Oct. 8-12 at the JW Marriott San Antonio Hill Country Resort and Spa in San Antonio, Texas.
Mark Breazzano, MD, discusses the future of retina specialists' practice within context of the ongoing pandemic.

Findings from the Archway trial showed that the PDS effect is long-lasting, consistent, and comparable to those seen with monthly injections.

Programming includes 16 EURETINA sessions; 22 symposia; 44 instructional courses; 85 hours of content, and more than 300 speakers.
Visual benefit is not seen during first 2 years of Protocol W study.

Dexamethasone implant, aflibercept offer a safe treatment option for diabetic macular edema.

Dr. Mark Breazzano discusses pandemic-related patient hesitancy and how retina specialists can help.

In response to a previously published article, three ophthalmologists present their views on intravitreal pharmacotherapy injections.

Results may represent a retinal degenerative change.

Investigators uncovered evidence that CaMKII could prove to be a desirable therapeutic target for vision preservation in conditions that damage the axons and somas of retinal ganglion cells.

Despite setbacks in DME, Adverum Biotechnologies said it will continue to develop ADVM-022 for wet AMD.

Long-term sustained-release therapy pays off in management of chronic, vision-threatening disease.

Outlook Therapeutics completes Phase 3 NORSE TWO safety and efficacy trial for ONS-5010, an investigational ophthalmic formulation of bevacizumab for treatment of wet AMD.

Mark Breazzano, MD, discusses the future of telehealth for retina specialists.

The U.S. FDA granted orphan drug designation to ADX-2191, methotrexate for intravitreal injection, as treatment for retinitis pigmentosa.

FDA will be asked to consider ONS-5010 for treating wet AMD.

If approved, Genentech’s treatment of faricimab will be the first and only medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.

New opportunities continue to develop in an evolving landscape.

With orphan drug and fast track status, Neurotech plans to seek FDA approval for its NT-501 Implant platform late next year.

Investigators have found that 78% of neovascular AMD cases have been detected using the PHP home monitoring system.

A team of investigators used corneal confocal microscopy to identify corneal nerve damage in cases of long COVID-19.

The American Society of Cataract and Refractive Surgery hosted its 2021 ASCRS and ASOA Annual Meeting July 23-27 in Las Vegas, Nevada.

Nanoscope Therapeutics Inc. has dosed its first patient in a late-stage Phase 2b trial of a gene therapy that delivers multi-characteristic opsin to retinal cells.